Page 151 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 151
Cl 123
O Ti 4 R
N H S O R O R
H N H S O OR SECTION 2.1
O PhCH 2 H N TiCl 4 N Aldol Addition and
HO R O H O H OH Condensation Reactions
OR R H H
Cl 4 Ti O OR
syn CH Ph anti
2
syn transition structure anti transition structure
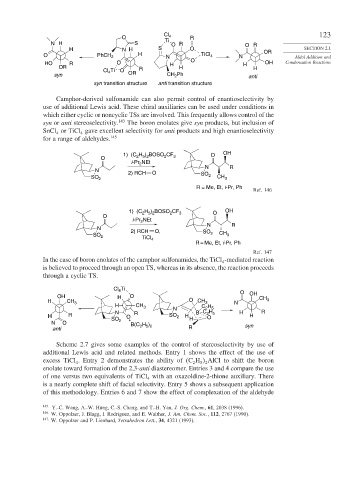
Camphor-derived sulfonamide can also permit control of enantioselectivity by
use of additional Lewis acid. These chiral auxiliaries can be used under conditions in
which either cyclic or noncyclic TSs are involved. This frequently allows control of the
syn or anti stereoselectivity. 143 The boron enolates give syn products, but inclusion of
SnCl or TiCl gave excellent selectivity for anti products and high enantioselectivity
4 4
for a range of aldehydes. 145
H ) BOSO CF OH
1) (C 2 5 2 2 3 O
O
i-Pr 2 NEt
N R
N 2) RCH O
SO 2 SO 2 CH 3
R = Me, Et, i-Pr, Ph
Ref. 146
1) (C H ) BOSO CF 3 O OH
2
2 5 2
O
i-Pr 2 NEt
N R
N
2) RCH O,
SO 2 TiCl SO 2 CH 3
4
R = Me, Et, i-Pr, Ph
Ref. 147
In the case of boron enolates of the camphor sulfonamides, the TiCl -mediated reaction
4
is believed to proceed through an open TS, whereas in its absence, the reaction proceeds
through a cyclic TS.
Cl Ti O
4
OH H O OH
H CH 3 O CH 3 N CH 3
H CH 3 N C 2 H 5
N R B C 2 H 5 H R
H R SO O SO 2 H H O H
N O 2 B(C H ) syn
anti 2 5 2 R
Scheme 2.7 gives some examples of the control of stereoselectivity by use of
additional Lewis acid and related methods. Entry 1 shows the effect of the use of
excess TiCl . Entry 2 demonstrates the ability of C H AlCl to shift the boron
2
4
5 2
enolate toward formation of the 2,3-anti diastereomer. Entries 3 and 4 compare the use
of one versus two equivalents of TiCl with an oxazoldine-2-thione auxiliary. There
4
is a nearly complete shift of facial selectivity. Entry 5 shows a subsequent application
of this methodology. Entries 6 and 7 show the effect of complexation of the aldehyde
145 Y.-C. Wang, A.-W. Hung, C.-S. Chang, and T.-H. Yan, J. Org. Chem., 61, 2038 (1996).
146 W. Oppolzer, J. Blagg, I. Rodriguez, and E. Walther, J. Am. Chem. Soc., 112, 2767 (1990).
147
W. Oppolzer and P. Lienhard, Tetrahedron Lett., 34, 4321 (1993).