Page 153 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 153
with TiCl using the camphor sultam auxiliary. Entry 8 is an example of the use of 125
4
excess diethylboron triflate to obtain the anti stereoisomer in a step in the synthesis of
epothilone. SECTION 2.1
These examples and those in Scheme 2.6 illustrate the key variables that determine Aldol Addition and
Condensation Reactions
the stereochemical outcome of aldol addition reactions using chiral auxiliaries. The
first element that has to be taken into account is the configuration of the ring system
that is used to establish steric differentiation. Then the nature of the TS, whether it is
acyclic, cyclic, or chelated must be considered. Generally for boron enolates, reaction
proceeds through a cyclic but nonchelated TS. With boron enolates, excess Lewis
acid can favor an acyclic TS by coordination with the carbonyl electrophile. Titanium
enolates appear to be somewhat variable but can be shifted to chelated TSs by use
of excess reagent and by auxiliaries such as oxazolidine-2-thiones that enhance the
tendency to chelation. Ultimately, all of the factors play a role in determining which
TS is favored.
2.1.5.6. Enantioselective Catalysis of the Aldol Addition Reaction. There are also
several catalysts that can effect enantioselective aldol addition. The reactions generally
involve enolate equivalents, such as silyl enol ethers, that are unreactive toward
the carbonyl component alone, but can react when activated by a Lewis acid. The
tryptophan-based oxazaborolidinone 15 has proven to be a useful catalyst. 148
O
N
H N O
Ts B
R
15
This catalyst induces preferential re facial attack on simple aldehydes, as indicated in
Figure 2.2. The enantioselectivity appears to involve the shielding of the si face by
the indole ring through a -stacking interaction.
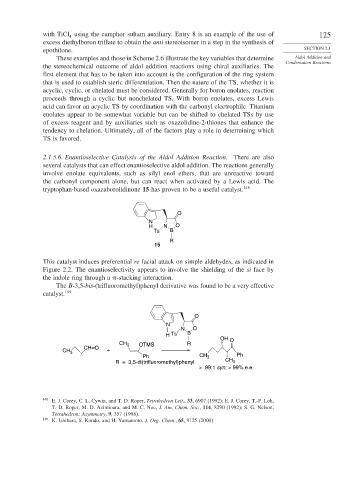
The B-3,5-bis-(trifluoromethyl)phenyl derivative was found to be a very effective
catalyst. 149
O
N
N O
H Ts B
OH O
CH 3 OTMS R
CH=O
CH 3 +
Ph CH 3 Ph
R = 3,5-di(trifluoromethyl)phenyl CH 3
> 99:1 syn; > 99% e.e.
148 E. J. Corey, C. L. Cywin, and T. D. Roper, Tetrahedron Lett., 33, 6907 (1992); E. J. Corey, T.-P. Loh,
T. D. Roper, M. D. Azimioara, and M. C. Noe, J. Am. Chem. Soc., 114, 8290 (1992); S. G. Nelson,
Tetrahedron: Asymmetry, 9, 357 (1998).
149
K. Ishihara, S. Kondo, and H. Yamamoto, J. Org. Chem., 65, 9125 (2000).