Page 154 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 154
126
CHAPTER 2 π π Interaction
Reactions of Carbon
Nucleophiles with
Carbonyl Compounds
re
Nuc
B
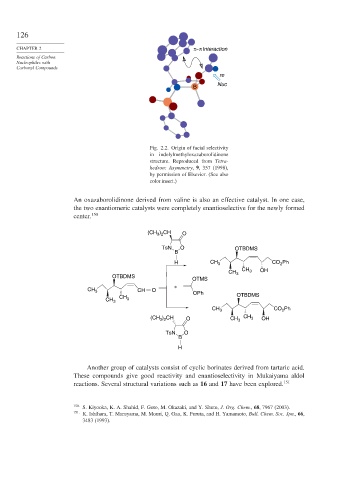
Fig. 2.2. Origin of facial selectivity
in indolylmethyloxazaborolidinone
structure. Reproduced from Tetra-
hedron: Asymmetry, 9, 357 (1998),
by permission of Elsevier. (See also
color insert.)
An oxazaborolidinone derived from valine is also an effective catalyst. In one case,
the two enantiomeric catalysts were completely enantioselective for the newly formed
center. 150
(CH ) CH O
3 2
TsN O OTBDMS
B
H CH 3 CO 2 Ph
CH OH
CH 3 3
OTBDMS
OTMS
+
CH 3 CH O OPh
CH OTBDMS
CH 3 3
CH 3 CO 2 Ph
(CH ) CH O CH 3 CH 3 OH
3 2
TsN O
B
H
Another group of catalysts consist of cyclic borinates derived from tartaric acid.
These compounds give good reactivity and enantioselectivity in Mukaiyama aldol
reactions. Several structural variations such as 16 and 17 have been explored. 151
150 S. Kiyooka, K. A. Shahid, F. Goto, M. Okazaki, and Y. Shuto, J. Org. Chem., 68, 7967 (2003).
151
K. Ishihara, T. Maruyama, M. Mouri, Q. Gao, K. Furuta, and H. Yamamoto, Bull. Chem. Soc. Jpn., 66,
3483 (1993).