Page 147 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 147
Ph Ph 119
ArSO N NSO Ar OH SECTION 2.1
2
2
B
CH O CO C(CH ) Aldol Addition and
2
3 3
Br Condensation Reactions
CH CH CO C(CH )
2
2
3 3
3
CH 3
Ar = 3,5-di(trifluoromethyl)phenyl 96:4 syn:anti, 75% e.e.
OH O
O 14
(CH ) CHCH O + (CH ) CH SPh
3 2
CH CH CSPh 3 2
2
3
CH 3 72%
97% e.e.
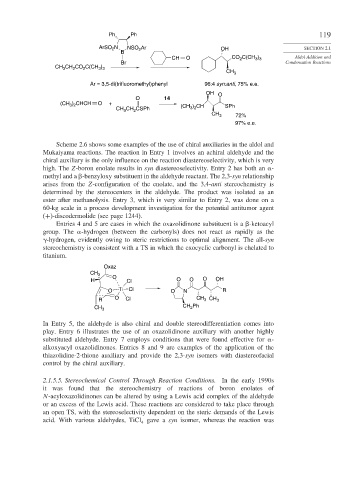
Scheme 2.6 shows some examples of the use of chiral auxiliaries in the aldol and
Mukaiyama reactions. The reaction in Entry 1 involves an achiral aldehyde and the
chiral auxiliary is the only influence on the reaction diastereoselectivity, which is very
high. The Z-boron enolate results in syn diastereoselectivity. Entry 2 has both an -
methyl and a -benzyloxy substituent in the aldehyde reactant. The 2,3-syn relationship
arises from the Z-configuration of the enolate, and the 3,4-anti stereochemistry is
determined by the stereocenters in the aldehyde. The product was isolated as an
ester after methanolysis. Entry 3, which is very similar to Entry 2, was done on a
60-kg scale in a process development investigation for the potential antitumor agent
(+)-discodermolide (see page 1244).
Entries 4 and 5 are cases in which the oxazolidinone substituent is a -ketoacyl
group. The -hydrogen (between the carbonyls) does not react as rapidly as the
-hydrogen, evidently owing to steric restrictions to optimal alignment. The all-syn
stereochemistry is consistent with a TS in which the exocyclic carbonyl is chelated to
titanium.
Oxaz
CH 3
H O Cl O O O OH
O Ti Cl O N R
O CH
R Cl 3 CH 3
CH Ph
CH 3 2
In Entry 5, the aldehyde is also chiral and double stereodifferentiation comes into
play. Entry 6 illustrates the use of an oxazolidinone auxiliary with another highly
substituted aldehyde. Entry 7 employs conditions that were found effective for -
alkoxyacyl oxazolidinones. Entries 8 and 9 are examples of the application of the
thiazolidine-2-thione auxiliary and provide the 2,3-syn isomers with diastereofacial
control by the chiral auxiliary.
2.1.5.5. Stereochemical Control Through Reaction Conditions. In the early 1990s
it was found that the stereochemistry of reactions of boron enolates of
N-acyloxazolidinones can be altered by using a Lewis acid complex of the aldehyde
or an excess of the Lewis acid. These reactions are considered to take place through
an open TS, with the stereoselectivity dependent on the steric demands of the Lewis
acid. With various aldehydes, TiCl gave a syn isomer, whereas the reaction was
4