Page 144 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 144
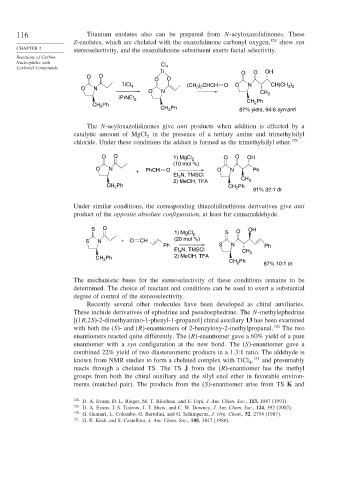
116 Titanium enolates also can be prepared from N-acyloxazolidinones. These
Z-enolates, which are chelated with the oxazolidinone carbonyl oxygen, 128 show syn
CHAPTER 2 stereoselectivity, and the oxazolidinone substituent exerts facial selectivity.
Reactions of Carbon
Nucleophiles with Cl
Carbonyl Compounds 4
Ti O O OH
O O
O O
TiCl 4 (CH ) CHCH O O N CH(CH )
3 2
O N 3 2
O N CH 3
i PrNEt 2
CH 2 Ph
CH Ph CH Ph 87% yield, 94:6 syn:anti
2
2
The N-acyloxazolidinones give anti products when addition is effected by a
catalytic amount of MgCl in the presence of a tertiary amine and trimethylsilyl
2
chloride. Under these conditions the adduct is formed as the trimethylsilyl ether. 129
O O 1) MgCl 2 O O OH
(10 mol %)
O N O N Ph
+ PhCH O
Et N, TMSCl
3
2) MeOH, TFA CH 3
CH Ph CH 2 Ph
2
91% 32:1 dr
Under similar conditions, the corresponding thiazolidinethione derivatives give anti
product of the opposite absolute configuration, at least for cinnamaldehyde.
S O OH
1) MgCl 2 S O
S N + O CH (20 mol %)
Ph S N Ph
Et 3 N, TMSCl
CH 3
CH Ph 2) MeOH, TFA
2
87% 10:1 dr
CH 2 Ph
The mechanistic basis for the stereoselectivity of these conditions remains to be
determined. The choice of reactant and conditions can be used to exert a substantial
degree of control of the stereoselectivity.
Recently several other molecules have been developed as chiral auxiliaries.
These include derivatives of ephedrine and pseudoephedrine. The N-methylephedrine
[(1R,2S)-2-dimethyamino-1-phenyl-1-propanol] chiral auxiliary 13 has been examined
with both the (S)- and (R)-enantiomers of 2-benzyloxy-2-methylpropanal. 130 The two
enantiomers reacted quite differently. The (R)-enantiomer gave a 60% yield of a pure
enantiomer with a syn configuration at the new bond. The (S)-enantiomer gave a
combined 22% yield of two diastereomeric products in a 1.3:1 ratio. The aldehyde is
known from NMR studies to form a chelated complex with TiCl , 131 and presumably
4
reacts through a chelated TS. The TS J from the (R)-enantiomer has the methyl
groups from both the chiral auxiliary and the silyl enol ether in favorable environ-
ments (matched pair). The products from the (S)-enantiomer arise from TS K and
128
D. A. Evans, D. L. Rieger, M. T. Bilodeau, and F. Urpi, J. Am. Chem. Soc., 113, 1047 (1991).
129
D. A. Evans, J. S. Tedrow, J. T. Shaw, and C. W. Downey, J. Am. Chem. Soc., 124, 392 (2002).
130 G. Gennari, L. Colombo, G. Bertolini, and G. Schimperna, J. Org. Chem., 52, 2754 (1987).
131
G. E. Keck and S. Castellino, J. Am. Chem. Soc., 108, 3847 (1986).