Page 139 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 139
OTMS 111
OTMS
(CH ) CH CH
3 2 3
(CH ) CH SECTION 2.1
CH 3 2
3
Aldol Addition and
OH OTBDMS OH OTBDMS
O OTBDMS O Condensation Reactions
CH(CH ) O CH )
(CH ) CH 3 2 (CH ) CH CH(CH 3 2
3 2
)
CH(CH 3 2 3 2
CH CH 3 CH
3 CH 3 95% 3 68%
95:5 syn:anti; CH 3 87:13 syn:anti ;
> 99:1 Felkin > 99:1 Felkin
OH OTBDMS
O OH OTBDMS
OTBDMS O
(CH ) CH CH(CH )
)
3 2 3 2 O CH (CH ) CH CH(CH 3 2
) 3 2
CH(CH 3 2
CH 3 CH 89% CH 3
3 CH 75%
CH 91:9 syn:anti; 3
70:30 syn:anti; 3
> 99:1 Felkin 87:13 Felkin-anti-Felkin
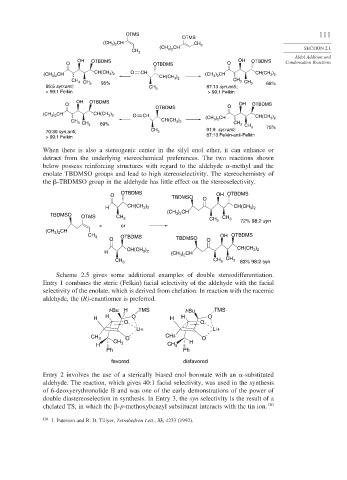
When there is also a stereogenic center in the silyl enol ether, it can enhance or
detract from the underlying stereochemical preferences. The two reactions shown
below possess reinforcing structures with regard to the aldehyde -methyl and the
enolate TBDMSO groups and lead to high stereoselectivity. The stereochemistry of
the -TBDMSO group in the aldehyde has little effect on the stereoselectivity.
OTBDMS
O OH OTBDMS
TBDMSO O
)
H CH(CH ) CH(CH 3 2
3 2
(CH ) CH
TBDMSO OTMS CH 3 3 2 CH CH 3
+ or 3 72% 98:2 syn
(CH 3 ) 2 CH
CH 3 OTBDMS OH OTBDMS
O TBDMSO O
) CH(CH )
3 2
H CH(CH 3 2 (CH ) CH
3 2
CH 3 CH 3 CH 3 83% 98:2 syn
Scheme 2.5 gives some additional examples of double stereodifferentiation.
Entry 1 combines the steric (Felkin) facial selectivity of the aldehyde with the facial
selectivity of the enolate, which is derived from chelation. In reaction with the racemic
aldehyde, the (R)-enantiomer is preferred.
t-Bu H TMS t-Bu TMS
H H O H H O
O O
Li+ Li+
CH 3 O CH3 O
CH 3 H
H CH 3
Ph Ph
favored disfavored
Entry 2 involves the use of a sterically biased enol boronate with an -substituted
aldehyde. The reaction, which gives 40:1 facial selectivity, was used in the synthesis
of 6-deoxyerythronolide B and was one of the early demonstrations of the power of
double diastereoselection in synthesis. In Entry 3, the syn selectivity is the result of a
chelated TS, in which the -p-methoxybenzyl substituent interacts with the tin ion. 120
120
I. Paterson and R. D. Tillyer, Tetrahedron Lett., 33, 4233 (1992).