Page 141 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 141
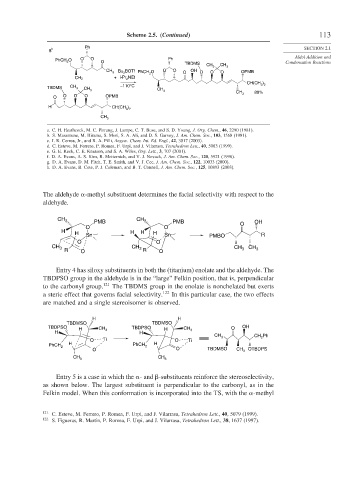
Scheme 2.5. (Continued) 113
Ph SECTION 2.1
8 h
Aldol Addition and
PhCH O O O Ph
2 O TBDMS Condensation Reactions
CH
3 CH 3
CH BOTf PhCH O O O OH OPMB
3 Bu 2 2 O O O O
CH 3 + i-Pr NEt
2
)
–110°C CH(CH 3 2
TBDMS CH 3 CH
3 CH 3
CH 80%
O O O O OPMB 3
H )
CH(CH 3 2
CH
3
a. C. H. Heathcock, M. C. Pirrung, J. Lampe, C. T. Buse, and S. D. Young, J. Org. Chem., 46, 2290 (1981).
b. S. Masamune, M. Hirama, S. Mori, S. A. Ali, and D. S. Garvey, J. Am. Chem. Soc., 103, 1568 (1981).
c. I. R. Correa, Jr., and R. A. Pilli, Angew. Chem. Int. Ed. Engl., 42, 3017 (2003).
d. C. Esteve, M. Ferrero, P. Romea, F. Urpi, and J. Vilarrasa, Tetrahedron Lett., 40, 5083 (1999).
e. G. E. Keck, C. E. Knutson, and S. A. Wiles, Org. Lett., 3, 707 (2001).
f. D. A. Evans, A. S. Kim, R. Metternich, and V. J. Novack, J. Am. Chem. Soc., 120, 5921 (1998).
g. D. A. Evans, D. M. Fitch, T. E. Smith, and V. J. Cee, J. Am. Chem. Soc., 122, 10033 (2000).
h. D. A. Evans, B. Cote, P. J. Coleman, and B. T. Connell, J. Am. Chem. Soc., 125, 10893 (2003).
The aldehyde -methyl substituent determines the facial selectivity with respect to the
aldehyde.
CH
PMB PMB O OH
CH 3 3
O O
H H H
H Sn H Sn PMBO R
O O
CH 3 CH 3 CH 3 CH 3
R O R O
Entry 4 has siloxy substituents in both the (titanium) enolate and the aldehyde. The
TBDPSO group in the aldehyde is in the “large” Felkin position, that is, perpendicular
to the carbonyl group. 121 The TBDMS group in the enolate is nonchelated but exerts
a steric effect that governs facial selectivity. 122 In this particular case, the two effects
are matched and a single stereoisomer is observed.
H H
TBDMSO TBDMSO
TBDPSO H TBDPSO H O OH
H CH 3 H CH 3
CH CH 2 Ph
O Ti O Ti 3
PhCH 2 H PhCH 2 H
O O TBDMSO CH 3 OTBDPS
CH 3 CH 3
Entry 5 is a case in which the - and -substituents reinforce the stereoselectivity,
as shown below. The largest substituent is perpendicular to the carbonyl, as in the
Felkin model. When this conformation is incorporated into the TS, with the -methyl
121 C. Esteve, M. Ferrero, P. Romea, F. Urpi, and J. Vilarrasa, Tetrahedron Lett., 40, 5079 (1999).
122
S. Figueras, R. Martin, P. Romea, F. Urpi, and J. Vilarrasa, Tetrahedron Lett., 38, 1637 (1997).