Page 142 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 142
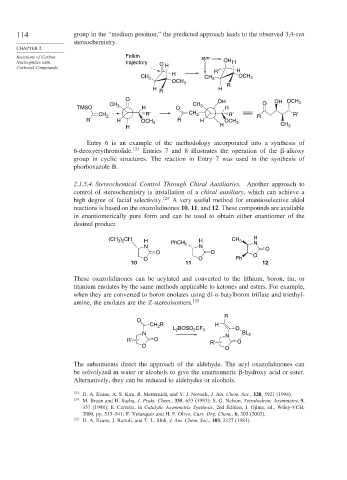
114 group in the “medium position,” the predicted approach leads to the observed 3,4-syn
stereochemistry.
CHAPTER 2
Reactions of Carbon Felkin syn
Nucleophiles with trajectory O H OH H
Carbonyl Compounds R′ H
H
CH 3 CH OCH 3
OCH 3 3
R
H R H
O OH
CH CH 3 O OH OCH 3
TMSO 3 H O H
R′ CH R′ R′
CH 2 2 R
R H OCH 3 R H OCH 3
H H CH 3
Entry 6 is an example of the methodology incorporated into a synthesis of
6-deoxyerythronolide. 123 Entries 7 and 8 illustrates the operation of the -alkoxy
group in cyclic structures. The reaction in Entry 7 was used in the synthesis of
phorboxazole B.
2.1.5.4. Stereochemical Control Through Chiral Auxiliaries. Another approach to
control of stereochemistry is installation of a chiral auxiliary, which can achieve a
high degree of facial selectivity. 124 A very useful method for enantioselective aldol
reactions is based on the oxazolidinones 10, 11, and 12. These compounds are available
in enantiomerically pure form and can be used to obtain either enantiomer of the
desired product.
(CH ) CH H PhCH H CH 3 H
3 2
N 2 N N O
O O
O O Ph O
10 11 12
These oxazolidinones can be acylated and converted to the lithium, boron, tin, or
titanium enolates by the same methods applicable to ketones and esters. For example,
when they are converted to boron enolates using di-n-butylboron triflate and triethyl-
amine, the enolates are the Z-stereoisomers. 125
R
O
CH R H
2
2
L 2 BOSO CF 3 O
N N BL 2
R′ O R′ O
O
O
The substituents direct the approach of the aldehyde. The acyl oxazolidinones can
be solvolyzed in water or alcohols to give the enantiomeric -hydroxy acid or ester.
Alternatively, they can be reduced to aldehydes or alcohols.
123
D. A. Evans, A. S. Kim, R. Metternich, and V. J. Novack, J. Am. Chem. Soc., 120, 5921 (1998).
124 M. Braun and H. Sacha, J. Prakt. Chem., 335, 653 (1993); S. G. Nelson, Tetrahedron: Asymmetry, 9,
357 (1998); E. Carreira, in Catalytic Asymmetric Synthesis, 2nd Edition, I. Ojima, ed., Wiley-VCH,
2000, pp. 513–541; F. Velazquez and H. F. Olivo, Curr. Org. Chem., 6, 303 (2002).
125
D. A. Evans, J. Bartoli, and T. L. Shih, J. Am. Chem. Soc., 103, 2127 (1981).