Page 140 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 140
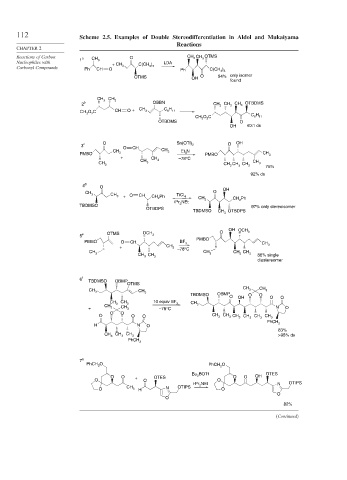
112 Scheme 2.5. Examples of Double Stereodifferentiation in Aldol and Mukaiyama
Reactions
CHAPTER 2
Reactions of Carbon 1 a CH O CH CH OTMS
3
3
Nucleophiles with 3 + CH C(CH ) LDA
Carbonyl Compounds Ph CH O 3 3 3 Ph C(CH ) 3
3
OTMS OH O 54% only isomer
found
CH 3 CH 3
2 b OBBN CH CH 3 CH 3 OTBDMS
3
6
CH O C CH O + CH 3 C H 11
2
3
O C C 6 H
2
CH 3 11
OTBDMS O
OH 40:1 ds
3 c O Sn(OTf) 2 O OH
O CH CH
PMBO CH 3 3 Et N PMBO CH 3
3
+ CH –78°C
CH 3
CH 3 CH CH 3
3 3 CH 3 CH 3 75%
92% ds
4 d O
O OH
CH 3 + O CH CH Ph TiCl 4 CH
CH 3
2
2
i Pr NEt 3 CH Ph
TBDMSO 3 97% only stereoisomer
OTBDPS
TBDMSO CH OTBDPS
3
OH OCH 3
O
5 e OTMS OCH 3
PMBO
PMBO O CH BF 3 CH
+ CH 3 –78°C 3
CH 3 CH CH 3 CH 3 CH 3
3 CH 3 86% single
diastereomer
6 f
TBDMSO OBMP
OTMS
CH
CH CH 3
CH 3
3 3 OBMP
TBDMSO O O
O OH O O
CH CH 10 equiv BF 3 CH 3
CH 3 3
+ 3 CH 3 –78°C N O
O O
O O O CH 3 CH 3 CH 3 CH 3 CH 3 CH 3
PhCH
H N O 2
83%
CH CH CH 3 >95% ds
3 3
PhCH
2
7 g
PhCH O PhCH O
2 2
BOTf OTES
Bu 2 OH
O O OTES O O
O + O O
i-Pr NEt N OTIPS
CH N OTIPS 2
O 3 H O
O
O
82%
(Continued)