Page 135 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 135
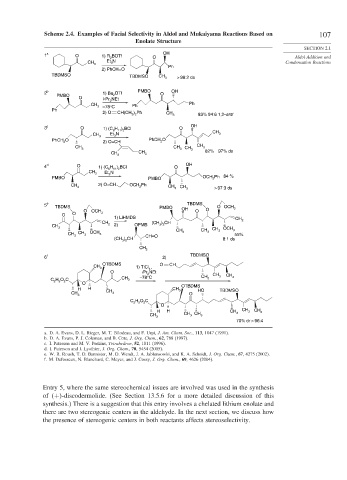
Scheme 2.4. Examples of Facial Selectivity in Aldol and Mukaiyama Reactions Based on 107
Enolate Structure
SECTION 2.1
1 a O 1) R BOTf OH
2 O Aldol Addition and
CH 3 Et N Condensation Reactions
3
2) PhCH=O Ph
TBDMSO CH
TBDMSO 3 > 98:2 ds
2 b 1) Bu OTf PMBO O OH
PMBO 2
O i-Pr NEt
2
CH Ph Ph
3 –78°C
Ph
2) O CH(CH ) Ph CH
2 2 3 83% 94:6 1,2-anti
3 c O 1) (C H ) BCl O OH
6 11 2 CH
CH 3 Et N 3
3
PhCH O PhCH O
2 2) O=CH 2
CH 3 CH 3 CH 3 CH 3
CH CH 3 82% 97% ds
3
4 d O 1) (C H ) BCl O OH
11 2
6
CH 3 Et N
PMBO 3 PMBO OCH Ph 84 %
2
CH 2) O=CH OCH Ph CH CH
3 2 3 3 > 97:3 ds
5 e TBDMS PMBO TBDMS O OCH
O OCH OH O O 3
O O 3
1) LiHMDS CH
CH (CH ) CH 2
CH 2 2) OPMB 3 2
3 CH CH CH 3 OCH 3
CH CH 3 OCH 3 3 3 55%
3 CH=O
(CH ) CH 8:1 ds
3 2
CH 3
6 f 2) TBDMSO
OTBDMS O CH
CH 1) TiCl ,
3 4
O iPr NEt
2 CH CH
CH –78°C CH 3 3
C H O C 3 3
2 5 2
O OTBDMS
H H CH HO TBDMSO
CH CH 3 3 O
3
C H O C
2 5 2
O
H H CH CH 3 CH 3
CH CH 3 CH 3 3
3
70% dr > 96:4
a. D. A. Evans, D. L. Rieger, M. T. Bilodeau, and F. Urpi, J. Am. Chem. Soc., 113, 1047 (1991).
b. D. A. Evans, P. J. Coleman, and B. Cote, J. Org. Chem., 62, 788 (1997).
c. I. Paterson and M. V. Perkins, Tetrahedron, 52, 1811 (1996).
d. I. Paterson and I. Lyothier, J. Org. Chem., 70, 5454 (2005).
e. W. R. Roush, T. D. Bannister, M. D. Wendt, J. A. Jablonsowki, and K. A. Scheidt, J. Org. Chem., 67, 4275 (2002).
f. M. Defosseux, N. Blanchard, C. Meyer, and J. Cossy, J. Org. Chem., 69, 4626 (2004).
Entry 5, where the same stereochemical issues are involved was used in the synthesis
of + -discodermolide. (See Section 13.5.6 for a more detailed discussion of this
synthesis.) There is a suggestion that this entry involves a chelated lithium enolate and
there are two stereogenic centers in the aldehyde. In the next section, we discuss how
the presence of stereogenic centers in both reactants affects stereoselectivity.