Page 136 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 136
108 OCH 3
CH 2
CH
CHAPTER 2 3
Reactions of Carbon PMBO CH 3 O O OTBDMS
Nucleophiles with CH 3 H
Carbonyl Compounds H Li
CH 3 H H O CH 3
O
CH 3
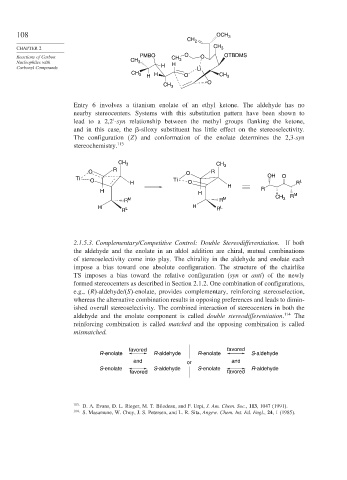
Entry 6 involves a titanium enolate of an ethyl ketone. The aldehyde has no
nearby stereocenters. Systems with this substitution pattern have been shown to
lead to a 2,2 -syn relationship between the methyl groups flanking the ketone,
and in this case, the -siloxy substituent has little effect on the stereoselectivity.
The configuration (Z) and conformation of the enolate determines the 2,3-syn
stereochemistry. 113
CH 3 CH 3
O R O R
Ti Ti OH O
O H O R L
H
H H R M
R M R M CH 3 R
H H L
R L R
2.1.5.3. Complementary/Competitive Control: Double Stereodifferentiation. If both
the aldehyde and the enolate in an aldol addition are chiral, mutual combinations
of stereoselectivity come into play. The chirality in the aldehyde and enolate each
impose a bias toward one absolute configuration. The structure of the chairlike
TS imposes a bias toward the relative configuration (syn or anti) of the newly
formed stereocenters as described in Section 2.1.2. One combination of configurations,
e.g., (R)-aldehyde/(S)-enolate, provides complementary, reinforcing stereoselection,
whereas the alternative combination results in opposing preferences and leads to dimin-
ished overall stereoselectivity. The combined interaction of stereocenters in both the
aldehyde and the enolate component is called double stereodifferentiation. 114 The
reinforcing combination is called matched and the opposing combination is called
mismatched.
favored favored
R-enolate R-aldehyde R-enolate S-aldehyde
and or and
S-enolate S-aldehyde S-enolate R-aldehyde
favored favored
113 D. A. Evans, D. L. Rieger, M. T. Bilodeau, and F. Urpi, J. Am. Chem. Soc., 113, 1047 (1991).
114
S. Masamune, W. Choy, J. S. Petersen, and L. R. Sita, Angew. Chem. Int. Ed. Engl., 24, 1 (1985).