Page 133 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 133
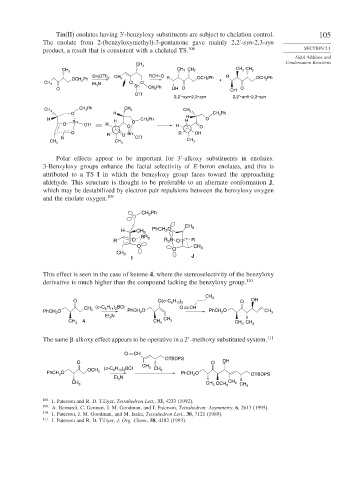
Tin(II) enolates having 3 -benzyloxy substituents are subject to chelation control. 105
The enolate from 2-(benzyloxymethyl)-3-pentanone gave mainly 2,2 -syn-2,3-syn
product, a result that is consistent with a chelated TS. 108 SECTION 2.1
Aldol Addition and
Condensation Reactions
CH 3
CH
CH 3 CH 3 CH 3
CH 3 3
RCH=O
Sn(OTf) 2 CH 3 R OCH 2 Ph R OCH Ph
OCH 2 Ph + 2
CH 3 Et 3 N O Sn O
O CH 2 Ph OH O OH O
OTf
2,2′-syn-2,3-syn 2,2′-anti -2,3-syn
CH 3 Ph CH 3
CH 3 CH 3
O H CH 2 Ph
H Sn H O CH 2 Ph H H O
O OTf H O H O
O Sn R OH
R O
R OTf CH
CH 3 CH 3 3
Polar effects appear to be important for 3 -alkoxy substituents in enolates.
3-Benzyloxy groups enhance the facial selectivity of E-boron enolates, and this is
attributed to a TS I in which the benzyloxy group faces toward the approaching
aldehyde. This structure is thought to be preferable to an alternate conformation J,
which may be destabilized by electron pair repulsions between the benzyloxy oxygen
and the enolate oxygen. 109
CH 2 Ph
CH 3
H CH 3 PhCH 2 O
BR 2
R O R 2 B O R
O CH 3
O
CH 3 J
I
This effect is seen in the case of ketone 4, where the stereoselectivity of the benzyloxy
derivative is much higher than the compound lacking the benzyloxy group. 110
CH
O O(c -C H ) 3 O OH
6
11 2
CH (c -C H ) BCl O CH
11 2
6
PhCH O 3 PhCH O PhCH O CH 3
2
2
2
Et 3 N CH
CH 3 4 CH 3 3 CH 3 CH 3
The same -alkoxy effect appears to be operative in a 2’-methoxy substituted system. 111
O CH
OTBDPS
O O OH
CH
OCH (c-C H ) BCl 3 CH 3
11 2
6
PhCH O 3 PhCH O OTBDPS
2
N 2
Et 3
CH 3 CH OCH 3 CH 3 CH 3
3
108
I. Paterson and R. D. Tillyer, Tetrahedron Lett., 33, 4233 (1992).
109
A. Bernardi, C. Gennari, J. M. Goodman, and I. Paterson, Tetrahedron: Asymmetry, 6, 2613 (1995).
110 I. Paterson, J. M. Goodman, and M. Isaka, Tetrahedron Lett., 30, 7121 (1989).
111
I. Paterson and R. D. Tillyer, J. Org. Chem., 58, 4182 (1993).