Page 131 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 131
103
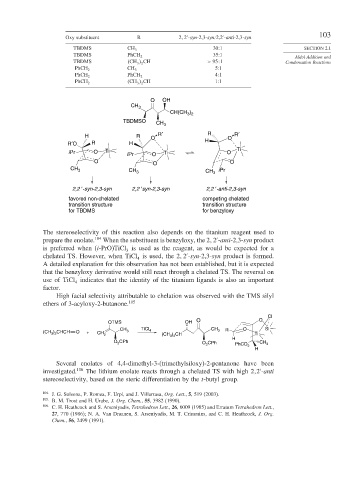
Oxy substituent R 2 2 -syn-2,3-syn:2,2 -anti-2,3-syn
TBDMS CH 3 30:1 SECTION 2.1
TBDMS 35:1
PhCH 2
Aldol Addition and
TBDMS CH 3 2 CH > 95 1 Condensation Reactions
5:1
PhCH 2 CH 3
4:1
PhCH 2 PhCH 2
CH 3 2 CH 1:1
PhCH 2
O OH
CH 3
)
CH(CH 3 2
TBDMSO CH 3
R′ R R′
H R O O
R′O R H H
i Pr O Ti i Pr O Ti O Ti
O O O
CH 3 i Pr
CH 3 CH 3
2,2 ′-syn-2,3-syn 2,2 ′syn-2,3-syn 2,2 ′-anti-2,3-syn
favored non-chelated competing chelated
transition structure transition structure
for TBDMS for benzyloxy
The stereoselectivity of this reaction also depends on the titanium reagent used to
prepare the enolate. 104 When the substituent is benzyloxy, the 2 2 -anti-2,3-syn product
is preferred when (i-PrO)TiCl is used as the reagent, as would be expected for a
3
chelated TS. However, when TiCl is used, the 2 2 -syn-2,3-syn product is formed.
4
A detailed explanation for this observation has not been established, but it is expected
that the benzyloxy derivative would still react through a chelated TS. The reversal on
use of TiCl indicates that the identity of the titanium ligands is also an important
4
factor.
High facial selectivity attributable to chelation was observed with the TMS silyl
ethers of 3-acyloxy-2-butanone. 105
Cl
OTMS OH O O
TiCl 4 CH 3 R O Si
(CH 3 ) 2 CHCH O + CH 2 (CH ) CH Ti
CH 3
3 2
O 2 CPh O CPh H
2 PhCO CH 3
2
H
Several enolates of 4,4-dimethyl-3-(trimethylsiloxy)-2-pentanone have been
investigated. 106 The lithium enolate reacts through a chelated TS with high 2 2 -anti
stereoselectivity, based on the steric differentiation by the t-butyl group.
104 J. G. Solsona, P. Romea, F. Urpi, and J. Villarrasa, Org. Lett., 5, 519 (2003).
105 B. M. Trost and H. Urabe, J. Org. Chem., 55, 3982 (1990).
106
C. H. Heathcock and S. Arseniyadis, Tetrahedron Lett., 26, 6009 (1985) and Erratum Tetrahedron Lett.,
27, 770 (1986); N. A. Van Draanen, S. Arseniyadis, M. T. Crimmins, and C. H. Heathcock, J. Org.
Chem., 56, 2499 (1991).