Page 126 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 126
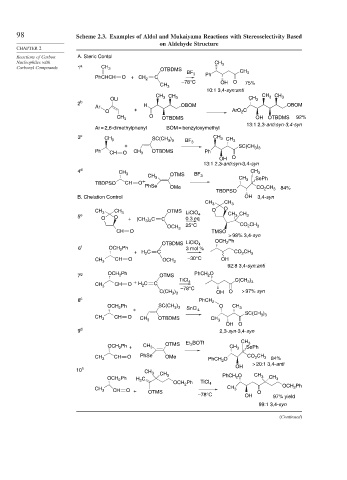
98 Scheme 2.3. Examples of Aldol and Mukaiyama Reactions with Stereoselectivity Based
on Aldehyde Structure
CHAPTER 2
Reactions of Carbon A. Steric Contol
Nucleophiles with CH 3
Carbonyl Compounds 1 a CH 3 OTBDMS
BF 3 Ph CH 3
PhCHCH O +CH 2 C
–78°C OH O 75%
CH 3
10:1 3,4-syn:anti
CH CH CH 3 CH 3
OLi 3 3 CH 3
2 b H OBOM OBOM
Ar
O + ArO C
2
CH 3 O OTBDMS OH OTBDMS 92%
13:1 2,3-anti:syn-3,4-syn
Ar = 2,6-dimethylphenyl BOM = benzyloxymethyl
3 c SC(CH ) CH 3 CH
CH 3 3 3 BF 3 3
+ SC(CH )
Ph CH O CH 3 OTBDMS Ph 3 3
OH O
13:1 2,3-anti:syn-3,4-syn
4 d CH
CH 3 OTMS 3
CH 3 BF 3 CH SePh
TBDPSO CH O + 3
PhSe OMe CO CH 84%
TBDPSO 2 3
B. Chelation Control OH 3,4-syn
CH 3 CH 3
CH 3 CH 3 OTMS LiClO O O
5 e O O + (CH ) C C 0.3 eq 4 CH 3 CH 3
3 2
25°C CO CH
OCH 3 2 3
CH O TMSO
> 98% 3,4-syn
OCH Ph
OTBDMS LiClO 4 2
6 f OCH Ph 3 mol %
2
+ H C C CO 2 CH 3
2
CH O –30°C OH
CH 3 OCH 3
92:8 3,4-syn:anti
7 g OCH Ph OTMS PhCH O
2
2
+ TiCl 4 C(CH )
3 3
CH 3 CH O H C C
2
–78°C
C(CH 3 ) 3 OH O > 97% syn
8 c PhCH 2
OCH 2 Ph SC(CH ) O CH 3
3 3
+ SnCl 4
)
SC(CH 3 3
CH 3 CH O CH 3 OTBDMS CH 3
OH O
9 d 2,3-syn-3,4-syn
3
2
OCH Ph + CH 3 OTMS Et BOTf CH 3 CH SePh
2
CH 3 CH O PhSe OMe PhCH 2 O CO 2 CH 3 84%
> 20:1 3,4-anti
OH
10 h CH 3 CH
2
OCH Ph H C 3 PhCH O CH 3 CH 3
2
2
OCH Ph TiCl 4
2
2
CH 3 OCH Ph
CH O + OTMS O
CH 3
–78°C OH 97% yield
99:1 3,4-syn
(Continued)