Page 129 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 129
PhCH 2 M n+ PhCH 2 101
O O OCH Ph
2
O OH SECTION 2.1
CH 3 CH 3 CH CO CH CH 3 CO CH 3 Aldol Addition and
2
H H 2 2 3 OH Condensation Reactions
CH 2 OTMS
OCH 3
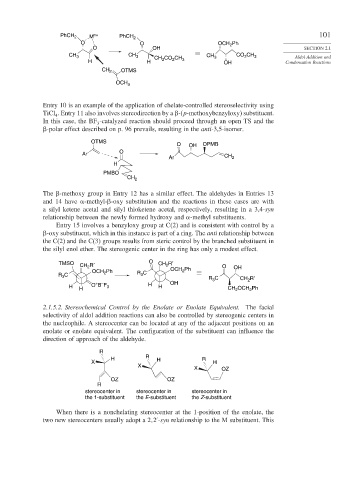
Entry 10 is an example of the application of chelate-controlled stereoselectivity using
TiCl . Entry 11 also involves stereodirection by a -(p-methoxybenzyloxy) substituent.
4
In this case, the BF -catalyzed reaction should proceed through an open TS and the
3
-polar effect described on p. 96 prevails, resulting in the anti-3,5-isomer.
OTMS
O OH OPMB
O
Ar
Ar CH 2
H
PMBO
CH 2
The -methoxy group in Entry 12 has a similar effect. The aldehydes in Entries 13
and 14 have -methyl- -oxy substitution and the reactions in these cases are with
a silyl ketene acetal and silyl thioketene acetal, respectively, resulting in a 3,4-syn
relationship between the newly formed hydroxy and -methyl substituents.
Entry 15 involves a benzyloxy group at C(2) and is consistent with control by a
-oxy substituent, which in this instance is part of a ring. The anti relationship between
the C(2) and the C(3) groups results from steric control by the branched substituent in
the silyl enol ether. The stereogenic center in the ring has only a modest effect.
TMSO R′ O CH 2 R′
CH 2 O OH
Ph
Ph OCH 2
OCH 2 R C
R C 3 R C CH 2 R′
3
3
+ –
H H O B F 3 H H OH CH 2 OCH Ph
2
2.1.5.2. Stereochemical Control by the Enolate or Enolate Equivalent. The facial
selectivity of aldol addition reactions can also be controlled by stereogenic centers in
the nucleophile. A stereocenter can be located at any of the adjacent positions on an
enolate or enolate equivalent. The configuration of the substituent can influence the
direction of approach of the aldehyde.
R
R
H H R
X H
X
X OZ
OZ OZ
R
stereocenter in stereocenter in stereocenter in
the 1-substituent the E-substituent the Z-substituent
When there is a nonchelating stereocenter at the 1-position of the enolate, the
two new stereocenters usually adopt a 2 2 -syn relationship to the M substituent. This