Page 127 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 127
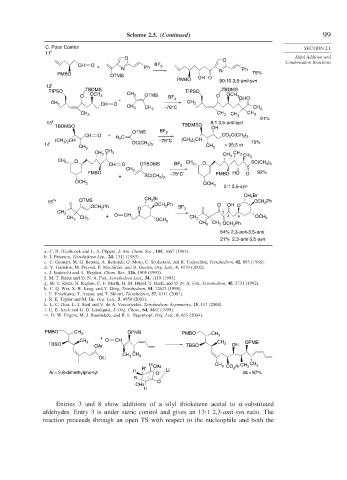
Scheme 2.3. (Continued) 99
C. Polar Control SECTION 2.1
11 i
O Aldol Addition and
O
CH O + BF 3 Condensation Reactions
N Ph N Ph
PMBO 75%
OTMS OH O
PMBO 90:10 3,5-anti:syn
12 j
TIPSO TBDMS CH TIPSO TBDMS
O OCH 3 3 OTMS BF O OCH 3
+ 3 OHO
CH O
CH 3 CH 3
CH 3 CH 3 –78°C CH 3
CH 3 CH 3 CH 3 CH 3
91%
13 k 8:1 3,5-anti:syn
TBDMSO TBDMSO OH
OTMS BF 3
CH O + C CO 2 C(CH )
3 3
(CH ) CH H 2 –78°C (CH ) CH 75%
3 2
)
14 l 3 2 OC(CH 3 3 CH > 95:5 dr
CH 3 3
CH 3 CH 3 CH CH 3 CH
CH 3 O 3 3 SC(CH )
CH O OTBDMS BF 3 CH 3 O 3 3
CH
PMBO 3 –78°C PMBO HO O 92%
+ SC(CH )
3 3
OCH 3 OCH 3 5:1 3,5-syn
CH Br
CH Br 2
15 m OTMS 2 OCH Ph
2
Ph OCH Ph O OH O
2
OCH 2 O BF 3
CH 3 2
CH 3 CH 3 + O CH CH 3 3 5 OCH 3
OCH 3
CH 3 CH 3 OCH 2 Ph
64% 2,3-anti-3,5-anti
21% 2,3-anti-3,5-syn
a. C. H. Heathcock and L. A. Flippin, J. Am. Chem. Soc., 105, 1667 (1983).
b. I. Paterson, Tetrahedron Lett., 24, 1311 (1983).
c. C. Gennari, M. G. Beretta, A. Bernardi, G. Moro, C. Scolastico, and R. Todeschini, Tetrahedron, 42, 893 (1986).
d. Y. Guindon, M. Prevost, P. Mochirian, and B. Guerin, Org. Lett., 4, 1019 (2002).
e. J. Ipaktschi and A. Heydari, Chem. Ber., 126, 1905 (1993).
f. M. T. Reetz and D. N. A. Fox, Tetrahedron Lett., 34, 1119 (1993).
g. M. T. Reetz, B. Raguse, C. F. Marth, H. M. Hügel, T. Bach, and D. N. A. Fox, Tetrahedron, 48, 5731 (1992).
h. C. Q. Wei, X. R. Jiang, and Y. Ding, Tetrahedron, 54, 12623 (1998).
i. F. Yokokawa, T. Asano, and T. Shioiri, Tetrahedron, 57, 6311 (2001).
j. R. E. Taylor and M. Jin, Org. Lett., 5, 4959 (2003).
k. L. C. Dias, L. J. Steil and V. de A. Vasconcelos, Tetrahedron: Asymmetry, 15, 147 (2004).
l. G. E. Keck and G. D. Lundquist, J. Org. Chem., 64, 4482 (1999).
m. D. W. Engers, M. J. Bassindale, and B. L. Pagenkopf, Org. Lett., 6, 663 (2004).
PMBO CH 3 OPMB PMBO CH
+ 3
CH 3 O CH CH OPMB
TBSO OAr TBSO 3 OH
CH 3 CH 3
OLi
H CH CH 3 CH 3
R' OAr 3 CO Ar
2
Ar = 2,6-dimethylphenyl H O Li ds > 97%
R
O
CH3
H
Entries 3 and 8 show additions of a silyl thioketene acetal to -substituted
aldehydes. Entry 3 is under steric control and gives an 13:1 2,3-anti-syn ratio. The
reaction proceeds through an open TS with respect to the nucleophile and both the