Page 130 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 130
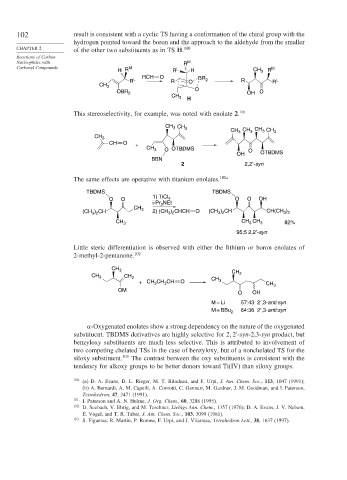
102 result is consistent with a cyclic TS having a conformation of the chiral group with the
hydrogen pointed toward the boron and the approach to the aldehyde from the smaller
CHAPTER 2 of the other two substituents as in TS H. 100
Reactions of Carbon
Nucleophiles with R M
Carbonyl Compounds M L M
H R R H CH 3 R
RCH O BR
R L R O 2 R R L
CH 3
OBR 2 O OH O
CH 3
H
This stereoselectivity, for example, was noted with enolate 2. 101
CH 3 CH 3
CH CH CH CH 3
3
3
3
CH 3
CH O +
CH 3 O OTBDMS O
OH OTBDMS
BBN
2 2,2′-syn
The same effects are operative with titanium enolates. 100a
TBDMS TBDMS
1) TiCl
O O 4 O O OH
i-Pr NEt
2
CH
)
(CH ) CH 3 2) (CH ) CHCH O (CH ) CH CH(CH 3 2
3 2
3 2
3 2
CH 3 CH CH 3 82%
3
95:5 2,2′-syn
Little steric differentiation is observed with either the lithium or boron enolates of
2-methyl-2-pentanone. 102
CH 3 CH
CH 3 CH 2 CH 3
+ CH CH CH O 3 CH 3
3
3
OM
O OH
M = Li 57:43 2′,3-anti:syn
M = BBu 2 64:36 2′,3-anti:syn
-Oxygenated enolates show a strong dependency on the nature of the oxygenated
substituent. TBDMS derivatives are highly selective for 2 2 -syn-2,3-syn product, but
benzyloxy substituents are much less selective. This is attributed to involvement of
two competing chelated TSs in the case of benzyloxy, but of a nonchelated TS for the
siloxy substituent. 103 The contrast between the oxy substituents is consistent with the
tendency for alkoxy groups to be better donors toward Ti(IV) than siloxy groups.
100 (a) D. A. Evans, D. L. Rieger, M. T. Bilodeau, and F. Urpi, J. Am. Chem. Soc., 113, 1047 (1991);
(b) A. Bernardi, A. M. Capelli, A. Comotti, C. Gennari, M. Gardner, J. M. Goodman, and I. Paterson,
Tetrahedron, 47, 3471 (1991).
101
I. Paterson and A. N. Hulme, J. Org. Chem., 60, 3288 (1995).
102 D. Seebach, V. Ehrig, and M. Teschner, Liebigs Ann. Chem., 1357 (1976); D. A. Evans, J. V. Nelson,
E. Vogel, and T. R. Taber, J. Am. Chem. Soc., 103, 3099 (1981).
103
S. Figueras, R. Martin, P. Romea, F. Urpi, and J. Vilarrasa, Tetrahedron Lett., 38, 1637 (1997).