Page 134 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 134
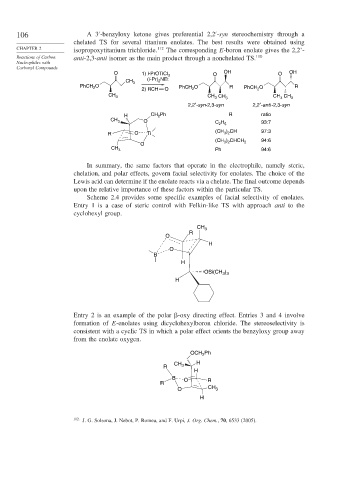
106 A3 -benzyloxy ketone gives preferential 2,2 -syn stereochemistry through a
chelated TS for several titanium enolates. The best results were obtained using
CHAPTER 2 isopropoxytitanium trichloride. 112 The corresponding E-boron enolate gives the 2,2’-
Reactions of Carbon anti-2,3-anti isomer as the main product through a nonchelated TS. 110
Nucleophiles with
Carbonyl Compounds
O 1) i-PrOTiCl 3 O OH O OH
(i-Pr) NEt
CH 3 2
PhCH O PhCH O R PhCH O R
2
2) RCH O 2 2
CH CH CH CH
CH 3 3 3 3 3
2,2′-syn-2,3-syn 2,2′-anti -2,3-syn
H CH Ph R ratio
2
CH 3 O C H 93:7
2 5
(CH ) CH 97:3
R O Ti 3 2
) CHCH 94:6
(CH 3 2 2
O
CH 3 Ph 94:6
In summary, the same factors that operate in the electrophile, namely steric,
chelation, and polar effects, govern facial selectivity for enolates. The choice of the
Lewis acid can determine if the enolate reacts via a chelate. The final outcome depends
upon the relative importance of these factors within the particular TS.
Scheme 2.4 provides some specific examples of facial selectivity of enolates.
Entry 1 is a case of steric control with Felkin-like TS with approach anti to the
cyclohexyl group.
CH 3
R
O
H
O
B
H
)
OSi(CH 3 3
H
Entry 2 is an example of the polar -oxy directing effect. Entries 3 and 4 involve
formation of E-enolates using dicyclohexylboron chloride. The stereoselectivity is
consistent with a cyclic TS in which a polar effect orients the benzyloxy group away
from the enolate oxygen.
Ph
OCH 2
H
R CH 3
H
B O R
R
O CH 3
H
112
J. G. Solsona, J. Nebot, P. Romea, and F. Urpi, J. Org. Chem., 70, 6533 (2005).