Page 137 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 137
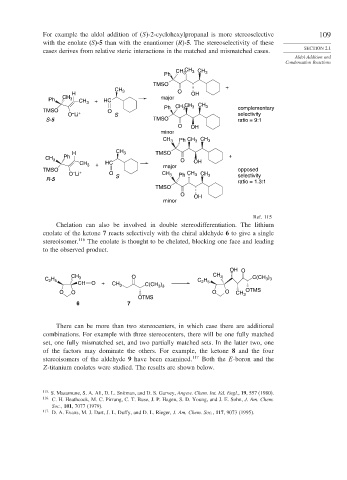
For example the aldol addition of (S)-2-cyclohexylpropanal is more stereoselective 109
with the enolate (S)-5 than with the enantiomer (R)-5. The stereoselectivity of these
SECTION 2.1
cases derives from relative steric interactions in the matched and mismatched cases.
Aldol Addition and
Condensation Reactions
CH 3 CH
Ph CH 3 3
TMSO
+
CH 3 O
H OH
CH major
Ph 3 CH 3 + HC
Ph CH 3 CH 3 CH 3 complementary
TMSO O
–
O Li + S selectivity
S-5 TMSO ratio = 9:1
O OH
minor
CH 3 Ph CH 3 CH 3
CH
H 3 TMSO
CH 3 Ph O +
CH 3 + HC major OH
TMSO opposed
–
O Li + O CH 3 Ph CH 3 CH 3 selectivity
R-5 S
ratio = 1.3:1
TMSO
O OH
minor
Ref. 115
Chelation can also be involved in double stereodifferentiation. The lithium
enolate of the ketone 7 reacts selectively with the chiral aldehyde 6 to give a single
stereoisomer. 116 The enolate is thought to be chelated, blocking one face and leading
to the observed product.
OH O
C H CH 3 O C H CH 3 C(CH )
3 3
2 5
CH O + CH 3 C(CH ) 2 5
3 3
O O O O CH 3 OTMS
OTMS
6 7
There can be more than two stereocenters, in which case there are additional
combinations. For example with three stereocenters, there will be one fully matched
set, one fully mismatched set, and two partially matched sets. In the latter two, one
of the factors may dominate the others. For example, the ketone 8 and the four
stereoisomers of the aldehyde 9 have been examined. 117 Both the E-boron and the
Z-titanium enolates were studied. The results are shown below.
115
S. Masamune, S. A. Ali, D. L. Snitman, and D. S. Garvey, Angew. Chem. Int. Ed. Engl., 19, 557 (1980).
116 C. H. Heathcock, M. C. Pirrung, C. T. Buse, J. P. Hagen, S. D. Young, and J. E. Sohn, J. Am. Chem.
Soc., 101, 7077 (1979).
117
D. A. Evans, M. J. Dart, J. L. Duffy, and D. L. Rieger, J. Am. Chem. Soc., 117, 9073 (1995).