Page 122 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 122
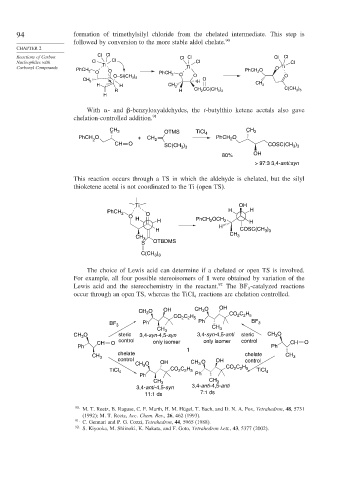
94 formation of trimethylsilyl chloride from the chelated intermediate. This step is
followed by conversion to the more stable aldol chelate. 90
CHAPTER 2
Cl Cl
Reactions of Carbon Cl Cl Cl Cl
Nucleophiles with Cl Ti Cl Cl Cl
Carbonyl Compounds Ti O Ti
PhCH 2 O PhCH O
O PhCH 2 2
O–Si(CH ) O O O
CH 3 3 O
3 H
H H CH 3 CH 3
)
R H CH 2 CC(CH ) C(CH 3 3
3 3
H
With - and -benzyloxyaldehydes, the t-butylthio ketene acetals also gave
chelation-controlled addition. 91
CH 3 OTMS TiCl CH 3
PhCH O + CH 4 PhCH O
2 2 2
CH O )
SC(CH 3 ) 3 COSC(CH 3 3
80% OH
> 97:3 3,4-anti:syn
This reaction occurs through a TS in which the aldehyde is chelated, but the silyl
thioketene acetal is not coordinated to the Ti (open TS).
Ti OH
H H
PhCH 2 O
O
H H PhCH OCH 2 H
2
H
H COSC(CH )
3 3
CH 3 CH 3
S OTBDMS
C(CH )
3 3
The choice of Lewis acid can determine if a chelated or open TS is involved.
For example, all four possible stereoisomers of 1 were obtained by variation of the
Lewis acid and the stereochemistry in the reactant. 92 The BF -catalyzed reactions
3
occur through an open TS, whereas the TiCl reactions are chelation controlled.
4
O OH CH 3 O OH
CH 3 CO C H
CO C H 2 2 5
2 2 5
BF 3 Ph Ph BF 3
CH 3 CH 3
3
CH 3 O steric 3,4-syn-4,5-syn 3,4-syn-4,5-anti steric CH O
control only isomer control
CH O only isomer CH O
Ph Ph
1
CH 3 chelate chelate CH 3
control CH O OH control
CH O OH 3
3
2 2 5
TiCl 4 CO C H CO C H TiCl 4
2 2 5
Ph Ph
CH 3 CH 3
3,4-anti- 4,5-syn 3,4-anti- 4,5-anti
11:1 ds 7:1 ds
90
M. T. Reetz, B. Raguse, C. F. Marth, H. M. Hügel, T. Bach, and D. N. A. Fox, Tetrahedron, 48, 5731
(1992); M. T. Reetz, Acc. Chem. Res., 26, 462 (1993).
91 C. Gennari and P. G. Cozzi, Tetrahedron, 44, 5965 (1988).
92
S. Kiyooka, M. Shiinoki, K. Nakata, and F. Goto, Tetrahedron Lett., 43, 5377 (2002).