Page 118 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 118
90 If the substituents are nonpolar, such as an alkyl or aryl group, the control is
exerted mainly by steric effects. In particular, for -substituted aldehydes, the Felkin
CHAPTER 2 TS model can be taken as the starting point for analysis, in combination with the
Reactions of Carbon cyclic TS. (See Section 2.4.1.3, Part A to review the Felkin model.) The analysis and
Nucleophiles with
Carbonyl Compounds prediction of the direction of the preferred reaction depends on the same principles
as for simple diastereoselectivity and are done by consideration of the attractive and
repulsive interactions in the presumed TS. In the Felkin model for nucleophilic addition
to carbonyl centers the larger -substituent is aligned anti to the approaching enolate
and yields the 3,4-syn product. If reaction occurs by an alternative approach, the
stereochemistry is reversed, and this is called an anti-Felkin approach.
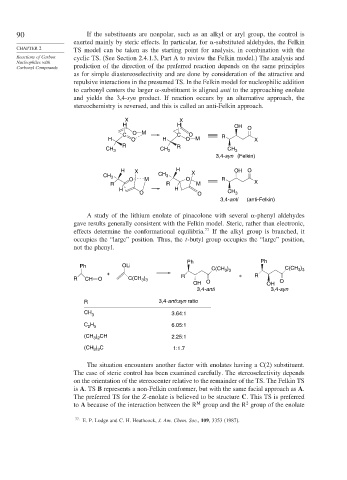
X X
H H OH O
C O M C O R
H O H O M X
R R
CH 3 CH 3 CH 3
3,4-syn (Felkin)
H X H OH O
CH 3 CH 3 X
O M O R
R R M X
H H
O O CH 3
3,4-anti (anti-Felkin)
A study of the lithium enolate of pinacolone with several -phenyl aldehydes
gave results generally consistent with the Felkin model. Steric, rather than electronic,
effects determine the conformational equilibria. 77 If the alkyl group is branched, it
occupies the “large” position. Thus, the t-butyl group occupies the “large” position,
not the phenyl.
Ph Ph
Ph OLi
C(CH ) C(CH )
3 3
3 3
+ + R
)
R CH O C(CH 3 3 R O
OH O OH
3,4-anti 3,4-syn
R 3,4-anti:syn ratio
CH 3.64:1
3
C H 6.05:1
2 5
(CH ) CH 2.25:1
3 2
(CH ) C 1:1.7
3 3
The situation encounters another factor with enolates having a C(2) substituent.
The case of steric control has been examined carefully. The stereoselectivity depends
on the orientation of the stereocenter relative to the remainder of the TS. The Felkin TS
is A.TS B represents a non-Felkin conformer, but with the same facial approach as A.
The preferred TS for the Z-enolate is believed to be structure C. This TS is preferred
2
M
to A because of the interaction between the R group and the R group of the enolate
77
E. P. Lodge and C. H. Heathcock, J. Am. Chem. Soc., 109, 3353 (1987).