Page 115 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 115
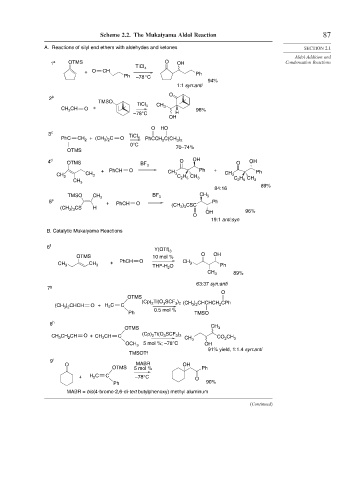
Scheme 2.2. The Mukaiyama Aldol Reaction 87
A. Reactions of silyl end ethers with aldehydes and ketones SECTION 2.1
Aldol Addition and
1 a OTMS O OH Condensation Reactions
TiCl 4
+ O CH Ph
Ph –78 °C
94%
1:1 syn:anti
O
2 b
TMSO TiCl
CH 3 CH O + 4 CH 3 96%
–78°C H
OH
O HO
3 c TiCl
PhC CH 2 + (CH ) C O 4 PhCCH 2 C(CH )
3 2
3 2
0°C 70–74%
OTMS
4 d OTMS BF 3 O OH O OH
+ PhCH O CH Ph + Ph
CH 3 CH 3 3 C H CH CH 3
2 5
CH 3 2 5 3 C H CH 3
89%
84:16
TMSO CH 3 BF 3 CH 3
5 e + PhCH O Ph
3 3
(CH ) CS H (CH ) CSC 96%
3 3
O OH
19:1 anti:syn
B. Catalytic Mukaiyama Reactions
6 f
Y(OTf) 3
OTMS 10 mol % O OH
CH + PhCH O CH 3
CH 3 3 THF-H O Ph
2
CH 3 89%
63:37 syn:anti
7 g
O
OTMS
(Cp) Ti(O SCF ) (CH ) CHCHCH CPh
(CH ) CHCH O + H C C 2 3 3 2 3 2 2
3 2 2
0.5 mol %
Ph TMSO
8 h
OTMS CH 3
3
CH CH CH O + CH CH C (Cp) 2 Ti(O SCF ) CH 3 CO CH 3
3 2
2
3
3
2
OCH 3 5 mol %; –78°C OH
91% yield, 1:1.4 syn:anti
TMSOTf
9 i
O MABR OH
OTMS 5 mol % Ph
+ H C C –78°C O
2
Ph 90%
MABR = bis(4-bromo-2,6-di-tert butylphenoxy) methyl aluminum
(Continued)