Page 114 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 114
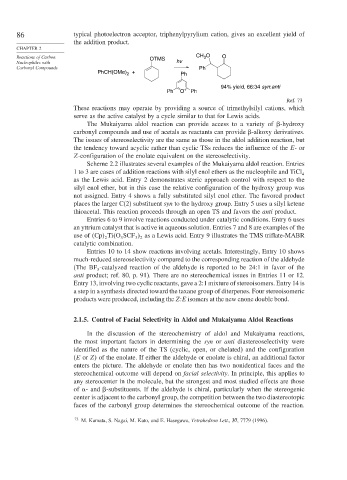
86 typical photoelectron acceptor, triphenylpyrylium cation, gives an excellent yield of
the addition product.
CHAPTER 2
Reactions of Carbon OTMS CH O O
3
Nucleophiles with hv
Carbonyl Compounds Ph
PhCH(OMe) + Ph
2
94% yield, 66:34 syn:anti
Ph O + Ph
Ref. 73
These reactions may operate by providing a source of trimethylsilyl cations, which
serve as the active catalyst by a cycle similar to that for Lewis acids.
The Mukaiyama aldol reaction can provide access to a variety of -hydroxy
carbonyl compounds and use of acetals as reactants can provide -alkoxy derivatives.
The issues of stereoselectivity are the same as those in the aldol addition reaction, but
the tendency toward acyclic rather than cyclic TSs reduces the influence of the E-or
Z-configuration of the enolate equivalent on the stereoselectivity.
Scheme 2.2 illustrates several examples of the Mukaiyama aldol reaction. Entries
1 to 3 are cases of addition reactions with silyl enol ethers as the nucleophile and TiCl 4
as the Lewis acid. Entry 2 demonstrates steric approach control with respect to the
silyl enol ether, but in this case the relative configuration of the hydroxy group was
not assigned. Entry 4 shows a fully substituted silyl enol ether. The favored product
places the larger C(2) substituent syn to the hydroxy group. Entry 5 uses a silyl ketene
thioacetal. This reaction proceeds through an open TS and favors the anti product.
Entries 6 to 9 involve reactions conducted under catalytic conditions. Entry 6 uses
an yttrium catalyst that is active in aqueous solution. Entries 7 and 8 are examples of the
use of Cp Ti O SCF as a Lewis acid. Entry 9 illustrates the TMS triflate-MABR
2 3 3 2
catalytic combination.
Entries 10 to 14 show reactions involving acetals. Interestingly, Entry 10 shows
much-reduced stereoselectivity compared to the corresponding reaction of the aldehyde
(The BF -catalyzed reaction of the aldehyde is reported to be 24:1 in favor of the
3
anti product; ref. 80, p. 91). There are no stereochemical issues in Entries 11 or 12.
Entry 13, involving two cyclic reactants, gave a 2:1 mixture of stereoisomers. Entry 14 is
a step in a synthesis directed toward the taxane group of diterpenes. Four stereoisomeric
products were produced, including the Z:E isomers at the new enone double bond.
2.1.5. Control of Facial Selectivity in Aldol and Mukaiyama Aldol Reactions
In the discussion of the stereochemistry of aldol and Mukaiyama reactions,
the most important factors in determining the syn or anti diastereoselectivity were
identified as the nature of the TS (cyclic, open, or chelated) and the configuration
(E or Z of the enolate. If either the aldehyde or enolate is chiral, an additional factor
enters the picture. The aldehyde or enolate then has two nonidentical faces and the
stereochemical outcome will depend on facial selectivity. In principle, this applies to
any stereocenter in the molecule, but the strongest and most studied effects are those
of - and -substituents. If the aldehyde is chiral, particularly when the stereogenic
center is adjacent to the carbonyl group, the competition between the two diastereotopic
faces of the carbonyl group determines the stereochemical outcome of the reaction.
73
M. Kamata, S. Nagai, M. Kato, and E. Hasegawa, Tetrahedron Lett., 37, 7779 (1996).