Page 112 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 112
84 Hindered bis-phenoxyaluminum derivatives are powerful cocatalysts for reactions
mediated by TMS triflate and are believed to act by promoting formation of
CHAPTER 2 60
trimethylsilyl cations by sequestering the triflate anion.
Reactions of Carbon
Nucleophiles with
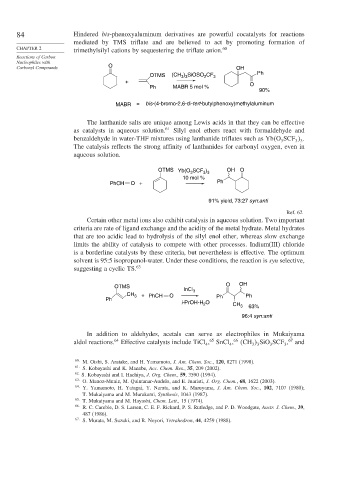
Carbonyl Compounds O OH
OTMS (CH ) SiOSO CF 3 Ph
3 3
2
+ O
Ph MABR 5 mol %
90%
MABR = bis-(4-bromo-2,6-di-tert-butylphenoxy)methylaluminum
The lanthanide salts are unique among Lewis acids in that they can be effective
as catalysts in aqueous solution. 61 Silyl enol ethers react with formaldehyde and
benzaldehyde in water-THF mixtures using lanthanide triflates such as Yb O SCF .
3 3
3
The catalysis reflects the strong affinity of lanthanides for carbonyl oxygen, even in
aqueous solution.
OTMS Yb(O SCF ) OH O
3 3 3
10 mol %
PhCH O + Ph
91% yield, 73:27 syn:anti
Ref. 62
Certain other metal ions also exhibit catalysis in aqueous solution. Two important
criteria are rate of ligand exchange and the acidity of the metal hydrate. Metal hydrates
that are too acidic lead to hydrolysis of the silyl enol ether, whereas slow exchange
limits the ability of catalysis to compete with other processes. Indium(III) chloride
is a borderline catalysts by these criteria, but nevertheless is effective. The optimum
solvent is 95:5 isopropanol-water. Under these conditions, the reaction is syn selective,
suggesting a cyclic TS. 63
O OH
OTMS
InCl 3
CH 3 + PhCH O Ph
Ph Ph
i-PrOH-H O CH 3 63%
2
96:4 syn:anti
In addition to aldehydes, acetals can serve as electrophiles in Mukaiyama
65
66
64
67
aldol reactions. Effective catalysts include TiCl , SnCl , CH SiO SCF , and
4
3
4
3
3 3
60
M. Oishi, S. Aratake, and H. Yamamoto, J. Am. Chem. Soc., 120, 8271 (1998).
61
S. Kobayashi and K. Manabe, Acc. Chem. Res., 35, 209 (2002).
62 S. Kobayashi and I. Hachiya, J. Org. Chem., 59, 3590 (1994).
63
O. Munoz-Muniz, M. Quintanar-Audelo, and E. Juaristi, J. Org. Chem., 68, 1622 (2003).
64
Y. Yamamoto, H. Yatagai, Y. Naruta, and K. Maruyama, J. Am. Chem. Soc., 102, 7107 (1980);
T. Mukaiyama and M. Murakami, Synthesis, 1043 (1987).
65 T. Mukaiyama and M. Hayashi, Chem. Lett., 15 (1974).
66 R. C. Cambie, D. S. Larsen, C. E. F. Rickard, P. S. Rutledge, and P. D. Woodgate, Austr. J. Chem., 39,
487 (1986).
67
S. Murata, M. Suzuki, and R. Noyori, Tetrahedron, 44, 4259 (1988).