Page 108 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 108
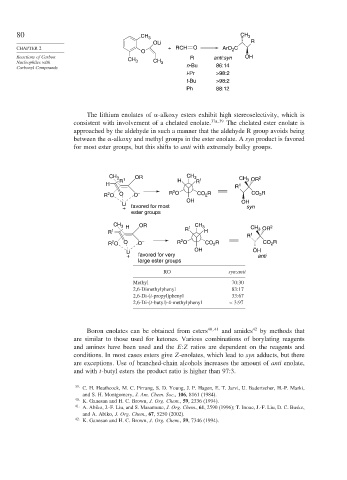
80 CH 3 CH 3
OLi R
CHAPTER 2 + RCH O ArO C
2
O
Reactions of Carbon CH R anti:syn OH
Nucleophiles with 3 CH 3
Carbonyl Compounds n-Bu 86:14
i-Pr >98:2
t-Bu >98:2
Ph 88:12
The lithium enolates of -alkoxy esters exhibit high stereoselectivity, which is
consistent with involvement of a chelated enolate. 37a 39 The chelated ester enolate is
approached by the aldehyde in such a manner that the aldehyde R group avoids being
between the -alkoxy and methyl groups in the ester enolate. A syn product is favored
for most ester groups, but this shifts to anti with extremely bulky groups.
CH 3 OR CH 3 2
3
R 1 H R 1 CH OR
H 1
R
2
2
R O O O – R O CO R CO R
2
2
OH OH
Li
+ favored for most syn
ester groups
CH 3 H OR 1 CH 3 2
3
R 1 R H CH OR
R 1
2
2
R O O O – R O CO 2 R CO R
2
OH OH
Li
+ favored for very anti
large ester groups
RO syn:anti
Methyl 70:30
2,6-Dimethylphenyl 83:17
2,6-Di-(i-propyl)phenyl 33:67
2,6-Di-(t-butyl)-4-methylphenyl < 3 97
Boron enolates can be obtained from esters 40 41 and amides 42 by methods that
are similar to those used for ketones. Various combinations of borylating reagents
and amines have been used and the E:Z ratios are dependent on the reagents and
conditions. In most cases esters give Z-enolates, which lead to syn adducts, but there
are exceptions. Use of branched-chain alcohols increases the amount of anti enolate,
and with t-butyl esters the product ratio is higher than 97:3.
39 C. H. Heathcock, M. C. Pirrung, S. D. Young, J. P. Hagen, E. T. Jarvi, U. Badertscher, H.-P. Marki,
and S. H. Montgomery, J. Am. Chem. Soc., 106, 8161 (1984).
40
K. Ganesan and H. C. Brown, J. Org. Chem., 59, 2336 (1994).
41 A. Abiko, J.-F. Liu, and S. Masamune, J. Org. Chem., 61, 2590 (1996); T. Inoue, J.-F. Liu, D. C. Buske,
and A. Abiko, J. Org. Chem., 67, 5250 (2002).
42
K. Ganesan and H. C. Brown, J. Org. Chem., 59, 7346 (1994).