Page 104 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 104
76 carbonyls over ketone carbonyls. The reaction works best with ketones having EWG
substituents such as alkynones and -haloketones. The reaction is thought to proceed
CHAPTER 2 through a cyclic intermediate that is stable until hydrolysis. This cyclic intermediate
Reactions of Carbon may be necessary to drive the normally unfavorable equilibrium of the addition step.
Nucleophiles with
Carbonyl Compounds OBu
BuO OBu
–
R′ OTMS CH 3 Li (BuO) Ti TMS Ti – OH
4
O + O H 2 O R CH O
O O O
+ R
(BuO) Ti R
RCR 4 R H R OBu R′
R′ R
R′
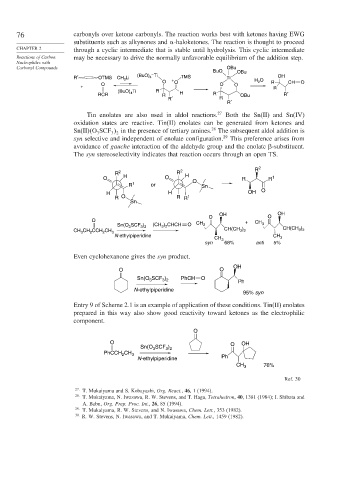
Tin enolates are also used in aldol reactions. 27 Both the Sn(II) and Sn(IV)
oxidation states are reactive. Tin(II) enolates can be generated from ketones and
28
Sn(II) O SCF in the presence of tertiary amines. The subsequent aldol addition is
3 2
3
syn selective and independent of enolate configuration. 29 This preference arises from
avoidance of gauche interaction of the aldehyde group and the enolate -substituent.
The syn stereoselectivity indicates that reaction occurs through an open TS.
R 2 R 2 H R 2
O H O O R R 1
R 1 or Sn
H H OH O
R O R R 1
Sn
OH OH
O O
O + CH
Sn(O SCF ) (CH ) CHCH O CH 3 3
3 2
3 2
3
)
CH CH CCH CH 3 CH(CH ) CH(CH 3 2
3 2
3
2
2
N-ethylpiperidine
CH 3 CH 3
syn 68% anti 5%
Even cyclohexanone gives the syn product.
OH
O O
SCF ) PhCH O
Sn(O 3 3 2 Ph
N-ethylpiperidine
95% syn
Entry 9 of Scheme 2.1 is an example of application of these conditions. Tin(II) enolates
prepared in this way also show good reactivity toward ketones as the electrophilic
component.
O
O O OH
Sn(O SCF )
3
3 2
PhCCH CH 3
2
N-ethylpiperidine Ph
CH 3 76%
Ref. 30
27 T. Mukaiyama and S. Kobayashi, Org. React., 46, 1 (1994).
28
T. Mukaiyama, N. Iwasawa, R. W. Stevens, and T. Haga, Tetrahedron, 40, 1381 (1984); I. Shibata and
A. Babu, Org. Prep. Proc. Int., 26, 85 (1994).
29 T. Mukaiyama, R. W. Stevens, and N. Iwasawa, Chem. Lett., 353 (1982).
30
R. W. Stevens, N. Iwasawa, and T. Mukaiyama, Chem. Lett., 1459 (1982).