Page 99 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 99
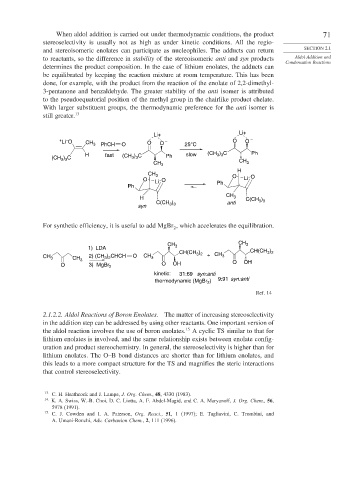
When aldol addition is carried out under thermodynamic conditions, the product 71
stereoselectivity is usually not as high as under kinetic conditions. All the regio-
and stereoisomeric enolates can participate as nucleophiles. The adducts can return SECTION 2.1
to reactants, so the difference in stability of the stereoisomeric anti and syn products Aldol Addition and
Condensation Reactions
determines the product composition. In the case of lithium enolates, the adducts can
be equilibrated by keeping the reaction mixture at room temperature. This has been
done, for example, with the product from the reaction of the enolate of 2,2-dimethyl-
3-pentanone and benzaldehyde. The greater stability of the anti isomer is attributed
to the pseudoequatorial position of the methyl group in the chairlike product chelate.
With larger substituent groups, the thermodynamic preference for the anti isomer is
still greater. 13
Li+ Li+
+ – O –
Li O CH 3 PhCH O O O – 25°C O
) C
H fast (CH ) C slow (CH 3 3 Ph
(CH ) C 3 3 Ph
3 3
CH 3 CH 3
H
CH 3 O
O Li O Li O
Ph Ph
CH
H 3 C(CH 3 3
)
C(CH ) anti
syn 3 3
For synthetic efficiency, it is useful to add MgBr , which accelerates the equilibration.
2
CH CH 3
1) LDA 3
CH(CH ) CH(CH )
3 2
CH 3 CH 3 2) (CH 3 2 O CH 3 3 2 + CH 3
) CHCH
O 3) MgBr 2 O OH O OH
kinetic: 31:69 syn:anti
thermodynamic (MgBr ) 9:91 syn:anti
2
Ref. 14
2.1.2.2. Aldol Reactions of Boron Enolates. The matter of increasing stereoselectivity
in the addition step can be addressed by using other reactants. One important version of
the aldol reaction involves the use of boron enolates. 15 A cyclic TS similar to that for
lithium enolates is involved, and the same relationship exists between enolate config-
uration and product stereochemistry. In general, the stereoselectivity is higher than for
lithium enolates. The O–B bond distances are shorter than for lithium enolates, and
this leads to a more compact structure for the TS and magnifies the steric interactions
that control stereoselectivity.
13
C. H. Heathcock and J. Lampe, J. Org. Chem., 48, 4330 (1983).
14 K. A. Swiss, W.-B. Choi, D. C. Liotta, A. F. Abdel-Magid, and C. A. Maryanoff, J. Org. Chem., 56,
5978 (1991).
15
C. J. Cowden and I. A. Paterson, Org. React., 51, 1 (1997); E. Tagliavini, C. Trombini, and
A. Umani-Ronchi, Adv. Carbanion Chem., 2, 111 (1996).