Page 96 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 96
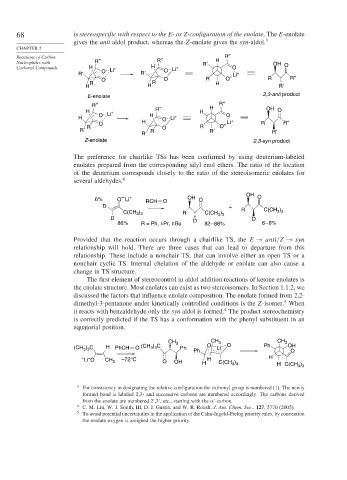
68 is stereospecific with respect to the E- or Z-configuration of the enolate. The E-enolate
gives the anti aldol product, whereas the Z-enolate gives the syn-aldol. 3
CHAPTER 2
Reactions of Carbon R″ R″
Nucleophiles with R″ R′ H OH O
Carbonyl Compounds H Li + H Li + O
R′ O R′ O Li +
O O R O – R R″
R R H
H H R′
E-enolate 2,3-anti product
R″ R″
H + R″ H H OH O
O Li H + O
H O Li
H Li + R″
O R O – R
R′ R R O R′ R′
R′
Z-enolate 2,3-syn product
The preference for chairlike TSs has been confirmed by using deuterium-labeled
enolates prepared from the corresponding silyl enol ethers. The ratio of the location
of the deuterium corresponds closely to the ratio of the stereoisomeric enolates for
several aldehydes. 4
OH
– +
6% O Li RCH O OH O O
D + R C(CH )
C(CH 3 ) 3 R C(CH ) 3 3
3 3
D D
86% R = Ph, i-Pr, t-Bu D 82 – 88% 6 – 8%
Provided that the reaction occurs through a chairlike TS, the E → anti/Z → syn
relationship will hold. There are three cases that can lead to departure from this
relationship. These include a nonchair TS, that can involve either an open TS or a
nonchair cyclic TS. Internal chelation of the aldehyde or enolate can also cause a
change in TS structure.
The first element of stereocontrol in aldol addition reactions of ketone enolates is
the enolate structure. Most enolates can exist as two stereoisomers. In Section 1.1.2, we
discussed the factors that influence enolate composition. The enolate formed from 2,2-
5
dimethyl-3-pentanone under kinetically controlled conditions is the Z-isomer. When
4
it reacts with benzaldehyde only the syn aldol is formed. The product stereochemistry
is correctly predicted if the TS has a conformation with the phenyl substituent in an
equatorial position.
CH CH
CH 3 3 O 3
) C
(CH ) C H PhCH O (CH 3 3 Ph Ph O Li Ph OH
3 3
O
+ – H H
Li O CH 3 –72°C O OH H C(CH ) H C(CH 3 3
)
3 3
3
For consistency in designating the relative configuration the carbonyl group is numbered (1). The newly
formed bond is labeled 2,3- and successive carbons are numbered accordingly. The carbons derived
from the enolate are numbered 2 ,3 , etc., starting with the -carbon.
4 C. M. Liu, W. J. Smith, III, D. J. Gustin, and W. R. Roush, J. Am. Chem. Soc., 127, 5770 (2005).
5
To avoid potential uncertainties in the application of the Cahn-Ingold-Prelog priority rules, by convention
the enolate oxygen is assigned the higher priority.