Page 101 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 101
BR 2 Cl 73
R
R O + R R +
C C O BR 2 CH 3 SECTION 2.1
H C CH 3 H CH 3 C H OBR 2 Aldol Addition and
OBR 2 H Condensation Reactions
H CH 3 H
E-enolate
R N: Z-enolate R N:
3
3
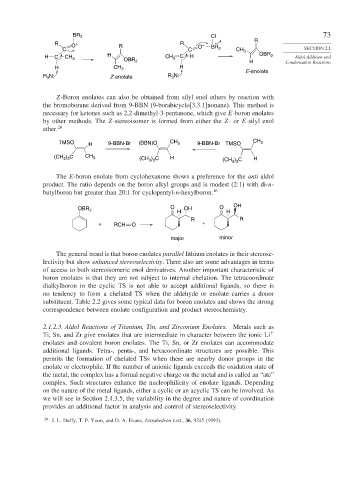
Z-Boron enolates can also be obtained from silyl enol ethers by reaction with
the bromoborane derived from 9-BBN (9-borabicyclo[3.3.1]nonane). This method is
necessary for ketones such as 2,2-dimethyl-3-pentanone, which give E-boron enolates
by other methods. The Z-stereoisomer is formed from either the Z-or E-silyl enol
ether. 20
TMSO H 9-BBN-Br (BBN)O CH 3 9-BBN-Br TMSO CH 3
(CH ) C CH 3 (CH ) C H (CH 3 3 H
) C
3 3
3 3
The E-boron enolate from cyclohexanone shows a preference for the anti aldol
product. The ratio depends on the boron alkyl groups and is modest (2:1) with di-n-
butylboron but greater than 20:1 for cyclopentyl-n-hexylboron. 16
OBR 2 O H OH O H OH
R R
+ RCH O +
major minor
The general trend is that boron enolates parallel lithium enolates in their stereose-
lectivity but show enhanced stereoselectivity. There also are some advantages in terms
of access to both stereoisomeric enol derivatives. Another important characteristic of
boron enolates is that they are not subject to internal chelation. The tetracoordinate
dialkylboron in the cyclic TS is not able to accept additional ligands, so there is
no tendency to form a chelated TS when the aldehyde or enolate carries a donor
substituent. Table 2.2 gives some typical data for boron enolates and shows the strong
correspondence between enolate configuration and product stereochemistry.
2.1.2.3. Aldol Reactions of Titanium, Tin, and Zirconium Enolates. Metals such as
Ti, Sn, and Zr give enolates that are intermediate in character between the ionic Li +
enolates and covalent boron enolates. The Ti, Sn, or Zr enolates can accommodate
additional ligands. Tetra-, penta-, and hexacoordinate structures are possible. This
permits the formation of chelated TSs when there are nearby donor groups in the
enolate or electrophile. If the number of anionic ligands exceeds the oxidation state of
the metal, the complex has a formal negative charge on the metal and is called an “ate”
complex. Such structures enhance the nucleophilicity of enolate ligands. Depending
on the nature of the metal ligands, either a cyclic or an acyclic TS can be involved. As
we will see in Section 2.1.3.5, the variability in the degree and nature of coordination
provides an additional factor in analysis and control of stereoselectivity.
20
J. L. Duffy, T. P. Yoon, and D. A. Evans, Tetrahedron Lett., 36, 9245 (1993).