Page 106 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 106
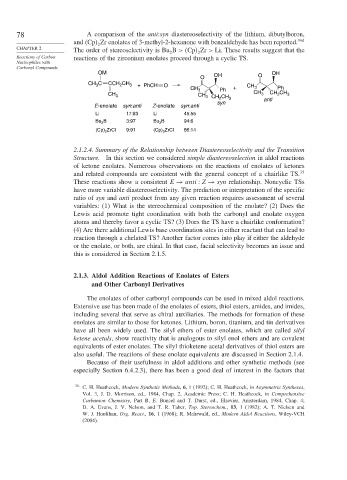
78 A comparison of the anti:syn diastereoselectivity of the lithium, dibutylboron,
and Cp Zr enolates of 3-methyl-2-hexanone with benzaldehyde has been reported. 34d
2
CHAPTER 2 The order of stereoselectivity is Bu B > Cp Zr > Li. These results suggest that the
2 2
Reactions of Carbon reactions of the zirconium enolates proceed through a cyclic TS.
Nucleophiles with
Carbonyl Compounds
OM OH OH
O O
CH C CCH CH 3 + PhCH O CH
2
3
CH 3 Ph + 3 Ph
CH 3 CH 3 CH CH 3 CH 3 CH CH 3
2
2
syn anti
E-enolate syn:anti Z-enolate syn:anti
Li 17:83 Li 45:55
Bu B 3:97 Bu B 94:6
2
2
ZrCl 9:91 (Cp) ZrCl 86:14
(Cp) 2 2
2.1.2.4. Summary of the Relationship between Diastereoselectivity and the Transition
Structure. In this section we considered simple diastereoselection in aldol reactions
of ketone enolates. Numerous observations on the reactions of enolates of ketones
and related compounds are consistent with the general concept of a chairlike TS. 35
These reactions show a consistent E → anti Z → syn relationship. Noncyclic TSs
have more variable diastereoselectivity. The prediction or interpretation of the specific
ratio of syn and anti product from any given reaction requires assessment of several
variables: (1) What is the stereochemical composition of the enolate? (2) Does the
Lewis acid promote tight coordination with both the carbonyl and enolate oxygen
atoms and thereby favor a cyclic TS? (3) Does the TS have a chairlike conformation?
(4) Are there additional Lewis base coordination sites in either reactant that can lead to
reaction through a chelated TS? Another factor comes into play if either the aldehyde
or the enolate, or both, are chiral. In that case, facial selectivity becomes an issue and
this is considered in Section 2.1.5.
2.1.3. Aldol Addition Reactions of Enolates of Esters
and Other Carbonyl Derivatives
The enolates of other carbonyl compounds can be used in mixed aldol reactions.
Extensive use has been made of the enolates of esters, thiol esters, amides, and imides,
including several that serve as chiral auxiliaries. The methods for formation of these
enolates are similar to those for ketones. Lithium, boron, titanium, and tin derivatives
have all been widely used. The silyl ethers of ester enolates, which are called silyl
ketene acetals, show reactivity that is analogous to silyl enol ethers and are covalent
equivalents of ester enolates. The silyl thioketene acetal derivatives of thiol esters are
also useful. The reactions of these enolate equivalents are discussed in Section 2.1.4.
Because of their usefulness in aldol additions and other synthetic methods (see
especially Section 6.4.2.3), there has been a good deal of interest in the factors that
35
C. H. Heathcock, Modern Synthetic Methods, 6, 1 (1992); C. H. Heathcock, in Asymmetric Syntheses,
Vol. 3, J. D. Morrison, ed., 1984, Chap. 2, Academic Press; C. H. Heathcock, in Comprehensive
Carbanion Chemistry, Part B, E. Buncel and T. Durst, ed., Elsevier, Amsterdam, 1984, Chap. 4;
D. A. Evans, J. V. Nelson, and T. R. Taber, Top. Stereochem., 13, 1 (1982); A. T. Nielsen and
W. J. Houlihan, Org. React., 16, 1 (1968); R. Mahrwald, ed., Modern Aldol Reactions, Wiley-VCH
(2004).