Page 109 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 109
OH 81
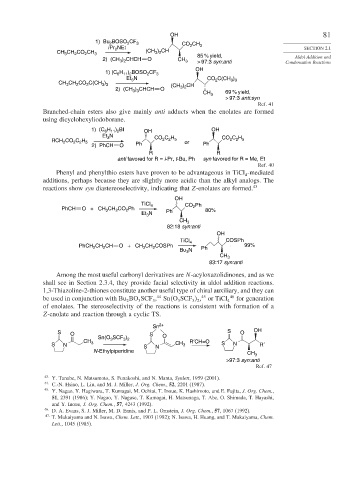
1) Bu BOSO CF 3 CO CH
2
2
i Pr NEt 2 3 SECTION 2.1
2
CH CH CO CH 3 (CH 3 ) 2 CH 85 % yield,
3
2
2
Aldol Addition and
2) (CH ) CHCH O CH 3 > 97:3 syn:anti Condensation Reactions
3 2
OH
H ) BOSO CF
1) (C 6 11 2 2 3
Et N CO C(CH )
3
2
3 3
CH CH CO C(CH ) (CH 3 ) 2 CH
3
3 3
2
2
2) (CH 3 ) 3 CHCH O
CH 3 69 % yield,
> 97:3 anti:syn
Ref. 41
Branched-chain esters also give mainly anti adducts when the enolates are formed
using dicyclohexyliodoborane.
1) (C H ) BI OH OH
6 11 2
Et 3 N CO C H CO C H
RCH 2 CO 2 C 2 H 5 Ph 2 2 5 or Ph 2 2 5
2) PhCH O
R R
anti favored for R = i-Pr, t-Bu, Ph syn favored for R = Me, Et
Ref. 40
Phenyl and phenylthio esters have proven to be advantageous in TiCl -mediated
4
additions, perhaps because they are slightly more acidic than the alkyl analogs. The
reactions show syn diastereoselectivity, indicating that Z-enolates are formed. 43
OH
TiCl 4 CO Ph
PhCH O + CH CH CO Ph 2 80%
2
2
3
Et N Ph
3
CH 3
82:18 syn:anti
OH
TiCl 4 COSPh
PhCH CH CH O + CH CH COSPh 99%
2
2
3
2
Bu N Ph
3
CH 3
83:17 syn:anti
Among the most useful carbonyl derivatives are N-acyloxazolidinones, and as we
shall see in Section 2.3.4, they provide facial selectivity in aldol addition reactions.
1,3-Thiazoline-2-thiones constitute another useful type of chiral auxiliary, and they can
45
44
be used in conjunction with Bu BO SCF , Sn O SCF , or TiCl 4 46 for generation
3
3 2
3
2
3
of enolates. The stereoselectivity of the reactions is consistent with formation of a
Z-enolate and reaction through a cyclic TS.
Sn 2+
S O S S O OH
Sn(O SCF ) O
3
3 2
CH 3 CH R′CH=O
S N S N 3 S N R′
N-Ethylpiperidine
CH 3
>97:3 syn:anti
Ref. 47
43 Y. Tanabe, N. Matsumoto, S. Funakoshi, and N. Manta, Synlett, 1959 (2001).
44
C.-N. Hsiao, L. Liu, and M. J. Miller, J. Org. Chem., 52, 2201 (1987).
45
Y. Nagao, Y. Hagiwara, T. Kumagai, M. Ochiai, T. Inoue, K. Hashimoto, and E. Fujita, J. Org. Chem.,
51, 2391 (1986); Y. Nagao, Y. Nagase, T. Kumagai, H. Matsunaga, T. Abe, O. Shimada, T. Hayashi,
and Y. Inoue, J. Org. Chem., 57, 4243 (1992).
46 D. A. Evans, S. J. Miller, M. D. Ennis, and P. L. Ornstein, J. Org. Chem., 57, 1067 (1992).
47
T. Mukaiyama and N. Isawa, Chem. Lett., 1903 (1982); N. Isawa, H. Huang, and T. Mukaiyama, Chem.
Lett., 1045 (1985).