Page 105 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 105
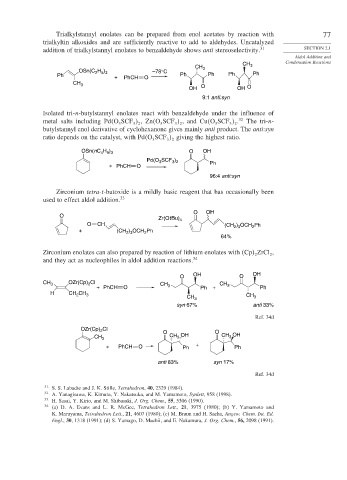
Trialkylstannyl enolates can be prepared from enol acetates by reaction with 77
trialkyltin alkoxides and are sufficiently reactive to add to aldehydes. Uncatalyzed
addition of trialkylstannyl enolates to benzaldehyde shows anti stereoselectivity. 31 SECTION 2.1
Aldol Addition and
CH 3 Condensation Reactions
CH 3
OSn(C H ) –78°C
2 5 3
Ph + PhCH O Ph Ph Ph Ph
CH 3
OH O OH O
9:1 anti:syn
Isolated tri-n-butylstannyl enolates react with benzaldehyde under the influence of
metal salts including Pd O SCF ,Zn O SCF , and Cu O SCF . 32 The tri-n-
3 2
3
3 2
3 2
3
3
butylstannyl enol derivative of cyclohexanone gives mainly anti product. The anti:syn
ratio depends on the catalyst, with Pd O SCF giving the highest ratio.
3
3 2
OSn(nC H ) O OH
4 9 3
Pd(O SCF )
3
3 2
+ PhCH O Ph
96:4 anti:syn
Zirconium tetra-t-butoxide is a mildly basic reagent that has occasionally been
used to effect aldol addition. 33
O OH
O
Zr(Ot Bu) 4
O CH (CH ) OCH Ph
2 3
2
) OCH Ph
+ (CH 2 3 2
64%
Zirconium enolates can also prepared by reaction of lithium enolates with Cp ZrCl ,
2
2
and they act as nucleophiles in aldol addition reactions. 34
OH OH
O O
CH 3 OZr(Cp) Cl CH CH
2
+ PhCH O 3 Ph + 3 Ph
H CH CH 3
2
CH 3 CH 3
syn 67% anti 33%
Ref. 34d
OZr(Cp) Cl O O
2
CH 3 CH 3 OH CH 3 OH
+ PhCH O Ph + Ph
anti 83% syn 17%
Ref. 34d
31 S. S. Labadie and J. K. Stille, Tetrahedron, 40, 2329 (1984).
32
A. Yanagisawa, K. Kimura, Y. Nakatsuka, and M. Yamamoto, Synlett, 958 (1998).
33 H. Sasai, Y. Kirio, and M. Shibasaki, J. Org. Chem., 55, 5306 (1990).
34
(a) D. A. Evans and L. R. McGee, Tetrahedron Lett., 21, 3975 (1980); (b) Y. Yamamoto and
K. Maruyama, Tetrahedron Lett., 21, 4607 (1980); (c) M. Braun and H. Sacha, Angew. Chem. Int. Ed.
Engl., 30, 1318 (1991); (d) S. Yamago, D. Machii, and E. Nakamura, J. Org. Chem., 56, 2098 (1991).