Page 94 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 94
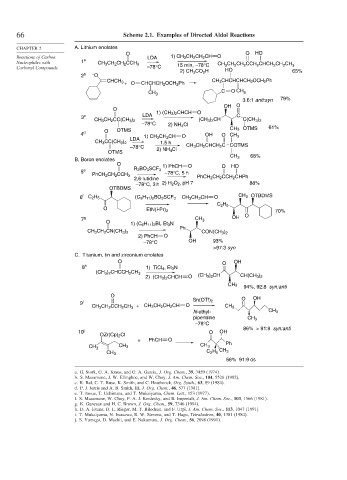
66 Scheme 2.1. Examples of Directed Aldol Reactions
CHAPTER 2 A. Lithium enolates
O O HO
Reactions of Carbon LDA 1) CH 3 CH 2 CH 2 CH O
Nucleophiles with 1 a CH 3 CH 2 CH 2 CCH 3 15 min, –78°C CH 3 CH CH CCH CHCH CH CH
Carbonyl Compounds –78°C 2 2 2 2 2 3
2) CH 3 CO 2 H HO 65%
2 b – O
CH 3 CHCHCHCH 2 OCH 2 Ph
CHCH 3 O CHCHCH 2 OCH 2 Ph
+
CH 3 C O CH 3
3.6:1 anti:syn 79%
OH O
O
1) (CH 3 ) 2 CHCH O
3 c LDA (CH 3 ) 2 CH C
CH 3 CH 2 CC(CH 3 ) 2 C(CH 3 ) 2
–78°C 2) NH 4 Cl
O OTMS CH3 OTMS 61%
4 d 1) CH 3 CH 2 CH O OH O CH 3
LDA
CH 3 CC(CH 3 ) 2 1.5 h CH 3 CH 2 CHCH 2 C COTMS
–78°C 2) NH 4 Cl
OTMS
CH 3 68%
B. Boron enolates
O
1) PhCH O O HO
5 e R 2 BO 3 SCF 3 –78°C, 5 h
PhCH 2 CH 2 CCH 3 PhCH 2 CH 2 CCH 2 CHPh
2,6-lutidine
–78°C, 3 h 2) H 2 O 2 , pH 7 88%
OTBDMS
6 f C 2 H 5 (C 5 H 11 ) 2 BO 3 SCF 3 CH 3 CH 2 CH O CH 3 OTBDMS
C 2 H 5
O
EtN(i-Pr) 2 70%
7 g CH 3 OH O
O 1) (C 6 H 11 ) 2 BI, Et 3 N
Ph
CH 3 CH 2 CN(CH 3 ) 2 CON(CH 3 ) 2
2) PhCH O
–78°C OH 93%
>97:3 syn
C. Titanium, tin and zirconium enolates
O O OH
8 h 1) TiCl 4 , Et 3 N
(CH 3 ) 2 CHCCH 2 CH 3 (CH 3 ) 2 CH
2) (CH 3 ) 2 CHCH O CH(CH 3 ) 2
CH3
94%, 92:8 syn:anti
O O OH
9 i Sn(OTf) 2
CH 3 CH 2 CCH 2 CH 3 + CH 3 CH 2 CH 2 CH O CH 3
N-ethyl- CH 3
piperidine CH 3
–78°C
86% > 91:9 syn:anti
10 j O OH
OZr(Cp) 2 Cl
+ PhCH O
CH 3 CH 3 CH 3 Ph
CH 3
C 2 H 5
CH 3
56% 91:9 ds
a. G. Stork, G. A. Kraus, and G. A. Garcia, J. Org. Chem., 39, 3459 (1974).
b. S. Masamune, J. W. Ellingboe, and W. Choy, J. Am. Chem. Soc., 104, 5526 (1982).
c. R. Bal, C. T. Buse, K. Smith, and C. Heathcock, Org. Synth., 63, 89 (1984).
d. P. J. Jerris and A. B. Smith, III, J. Org. Chem., 46, 577 (1981).
e. T. Inoue, T. Uchimaru, and T. Mukaiyama, Chem. Lett., 153 (1977).
f. S. Masamune, W. Choy, F. A. J. Kerdesky, and B. Imperiali, J. Am. Chem. Soc., 103, 1566 (1981).
g. K. Ganesan and H. C. Brown, J. Org. Chem., 59, 7346 (1994).
h. D. A. Evans, D. L. Rieger, M. T. Bilodeau, and F. Urpi, J. Am. Chem. Soc., 113, 1047 (1991).
i. T. Mukaiyama, N. Iwasawa, R. W. Stevens, and T. Hagu, Tetrahedron, 40, 1381 (1984).
j. S. Yamago, D. Machii, and E. Nakamura, J. Org. Chem., 56, 2098 (1991).