Page 89 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 89
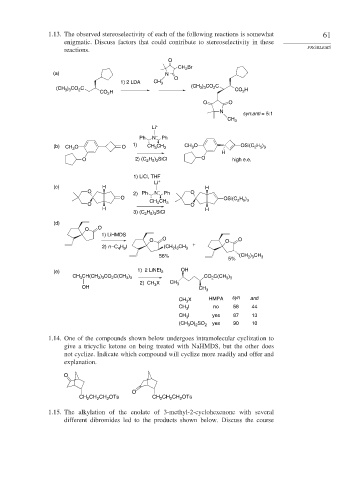
1.13. The observed stereoselectivity of each of the following reactions is somewhat 61
enigmatic. Discuss factors that could contribute to stereoselectivity in these
reactions. PROBLEMS
O
Br
CH 2
(a) N
O
1) 2 LDA CH 3
) CO C
) CO C (CH 3 3 2
(CH 3 3 2 CO H
CO H 2
2
O O
N
syn:anti = 5:1
CH 3
Li +
Ph N – Ph
(b) CH O O 1) CH CH 3 CH O OSi(C H )
2 5 3
3
3
3
H
O 2) (C H ) SiCl O high e.e.
2 5 3
1) LiCl, THF
Li +
(c) H H
O 2) Ph N – Ph O
O OSi(C H )
2 5 3
O CH 3 CH 3 O
H H
3) (C H ) SiCl
2 5 3
(d)
O O
1) LiHMDS
O O O O
+
2) n –C H I (CH ) CH 3
2 3
4 9
56% (CH ) CH 3
2 3
5%
(e) 1) 2 LiNEt 2 OH
CH(CH ) CO C(CH ) CO C(CH )
CH 3 2 3 2 3 3 2 3 3
2) CH X CH 3
OH 3 CH 3
CH X HMPA syn anti
3
CH I no 56 44
3
I yes 87 13
CH 3
(CH O) SO 2 yes 90 10
2
3
1.14. One of the compounds shown below undergoes intramolecular cyclization to
give a tricyclic ketone on being treated with NaHMDS, but the other does
not cyclize. Indicate which compound will cyclize more readily and offer and
explanation.
O
O
CH CH CH OTs CH CH CH OTs
2
2
2
2
2
2
1.15. The alkylation of the enolate of 3-methyl-2-cyclohexenone with several
different dibromides led to the products shown below. Discuss the course