Page 87 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 87
O O O O 59
(d)
(CH 3 O) 2 PCH 2 C(CH 2 ) 4 CH 3 (CH 3 O) 2 PCH 2 CCH 3
PROBLEMS
(e) PhCH 2 CH 2 CHCO 2 C 2 H 5 PhCH 2 CO 2 C 2 H 5
Ph
(f) O
O CH 3 CH CHCO 2 CH 3
CH 3
O
(g) CH 3
O CN NCCH 2 CO 2 C 2 H 5
O
(h) OCH 2 CH CH 2 OH
O HO
O
O HO
(i) CH 3 CH 3
CCH 2 CH 2 C CH 2 CCH 2 CO 2 CH 2 CH 3
O CH 3 O
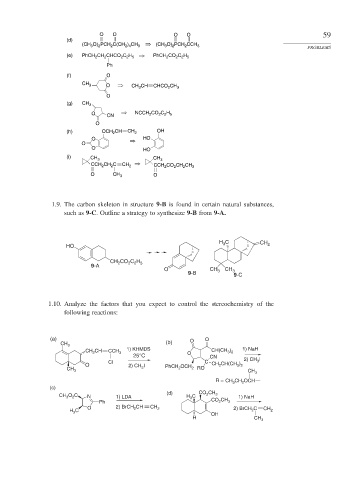
1.9. The carbon skeleton in structure 9-B is found in certain natural substances,
such as 9-C. Outline a strategy to synthesize 9-B from 9-A.
H C CH
3
HO 2
CH CO C H
2
2 2 5
9-A
O CH 3 CH 3
9-B 9-C
1.10. Analyze the factors that you expect to control the stereochemistry of the
following reactions:
(a) O O
CH 3 (b)
CH CH CCH 3 1) KHMDS O CH(CH ) 1) NaH
3 2
2
25°C CN
Cl C 2) CH 3 I
O 2) CH I PhCH OCH CH 2 CH(CH )
3 2
CH 3 3 2 2 RO CH 3
R = CH CH OCH
3 2
(c)
(d) CO CH
O C 2 3
CH 3 2 N 1) LDA H C 1) NaH
3
Ph CO CH 3
2
O 2) BrCH CH CH C CH
H C 2 2 2) BrCH 2 2
3
H OH CH
3