Page 90 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 90
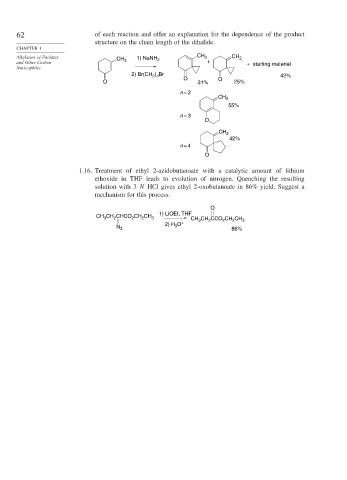
62 of each reaction and offer an explanation for the dependence of the product
structure on the chain length of the dihalide.
CHAPTER 1
Alkylation of Enolates 1) NaNH CH 3 CH 2
CH 3 2
and Other Carbon + + starting material
Nucleophiles
) Br
2) Br(CH 2 n 42%
O O
O 31% 25%
n = 2
CH 2
55%
n = 3
O
CH 2
42%
n = 4
O
1.16. Treatment of ethyl 2-azidobutanoate with a catalytic amount of lithium
ethoxide in THF leads to evolution of nitrogen. Quenching the resulting
solution with 3 N HCl gives ethyl 2-oxobutanoate in 86% yield. Suggest a
mechanism for this process.
O
1) LiOEt, THF
CH CH CHCO CH CH 3 CH CH CCO CH CH 3
2
2
3
2
2
3
2
2
+
O
N 3 2) H 3 86%