Page 86 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 86
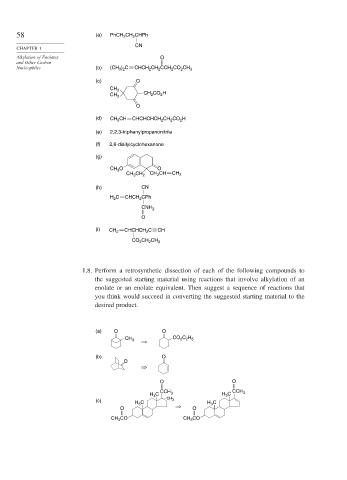
58 (a) PhCH 2 CH 2 CHPh
CN
CHAPTER 1
Alkylation of Enolates O
and Other Carbon
Nucleophiles (b) (CH 3 ) 2 C CHCH 2 CH 2 CCH 2 CO 2 CH 3
(c) O
CH 3
CH 2 CO 2 H
CH 3
O
(d) CH 3 CH CHCHCHCH 2 CH 2 CO 2 H
(e) 2,2,3-triphenylpropanonitrile
(f) 2,6-diallylcyclohexanone
(g)
CH 3 O O
CH 2 CH
CH 3 CH 2 CH 2
(h) CN
H 2 C CHCH 2 CPh
CNH 2
O
(i) CHCHCH 2 C CH
CH 2
CO 2 CH 2 CH 3
1.8. Perform a retrosynthetic dissection of each of the following compounds to
the suggested starting material using reactions that involve alkylation of an
enolate or an enolate equivalent. Then suggest a sequence of reactions that
you think would succeed in converting the suggested starting material to the
desired product.
(a) O O
CH 3 CO 2 C 2 H 5
(b) O
O
O O
H 3 C CCH 3 H 3 C CCH 3
(c) CH 3
H 3 C H 3 C
O O
CH 3 CO CH 3 CO