Page 84 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 84
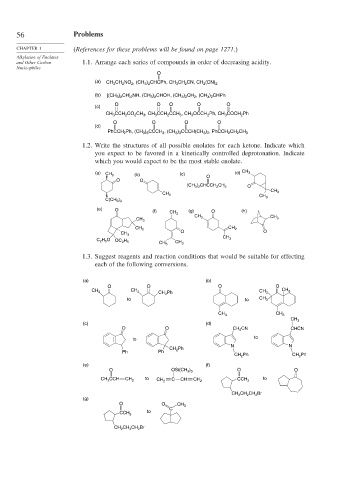
56 Problems
CHAPTER 1 (References for these problems will be found on page 1271.)
Alkylation of Enolates
and Other Carbon 1.1. Arrange each series of compounds in order of decreasing acidity.
Nucleophiles
O
(a) CH 3 CH 2 NO 2 , (CH 3 ) 2 CHCPh, CH 3 CH 2 CN, CH 2 (CN) 2
(b) [(CH 3 ) 2 CH] 2 NH, (CH 3 ) 2 CHOH, (CH 3 ) 2 CH 2 , (CH 3 ) 2 CHPh
O O O O O
(c)
CH 3 CCH 2 CO 2 CH 3 , CH 3 CCH 2 CCH 3 , CH 3 OCCH 2 Ph, CH 3 COCH 2 Ph
O O O O
(d)
PhCCH 2 Ph, (CH 3 ) 3 CCCH 3 , (CH 3 ) 3 CCCH(CH 3 ) 2 , PhCCH 2 CH 2 CH 3
1.2. Write the structures of all possible enolates for each ketone. Indicate which
you expect to be favored in a kinetically controlled deprotonation. Indicate
which you would expect to be the most stable enolate.
(a) (c) (d) CH 3
CH 3 (b)
O
O O
(CH 3 ) 2 CHCCH 2 CH 3 O
CH 3
CH 3
CH 3
C(CH 3 ) 3
(e) O (f) CH 3 (g) O (h)
CH 3 CH 3
CH 3
CH 3 CH 2
O O
CH 3
C 2 H 5 O OC 2 H 5 CH 3
CH 3 CH 3
1.3. Suggest reagents and reaction conditions that would be suitable for effecting
each of the following conversions.
(a) (b)
O O O O
CH 2 Ph CH 3
CH 3 CH 3 CH 3
to to CH 3
CH 3 CH 3
CH 3
(c) (d)
O O CH 2 CN CHCN
to
to
N N
CH 2 Ph
Ph Ph
CH 2 Ph CH 2 Ph
(e) (f)
O OSi(CH 3 ) 3 O O
CH 3 CCH CH 2 to C CH to
CH 2 CH 2 CCH 3
CH 2 CH 2 CH 2 Br
(g)
O O CH 3
C
to
CCH 3
CH 2 CH 2 CH 2 Br