Page 79 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 79
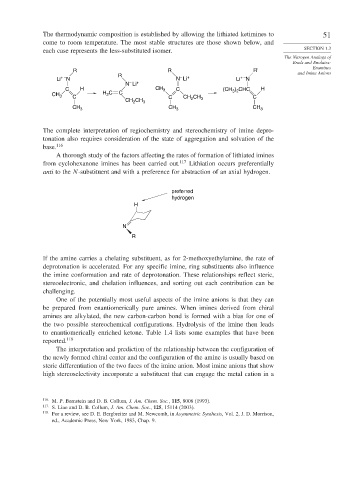
The thermodynamic composition is established by allowing the lithiated ketimines to 51
come to room temperature. The most stable structures are those shown below, and
each case represents the less-substituted isomer. SECTION 1.3
The Nitrogen Analogs of
Enols and Enolates:
Enamines
R R R' and Imine Anions
R +
–
Li + – N N Li Li + – N
–
N Li +
C H CH 3 C (CH ) CHC H
3 2
H C C
CH 3 2
C C CH 2 CH C
CH CH 3 3
2
CH 3 CH 3 CH 3
The complete interpretation of regiochemistry and stereochemistry of imine depro-
tonation also requires consideration of the state of aggregation and solvation of the
base. 116
A thorough study of the factors affecting the rates of formation of lithiated imines
from cyclohexanone imines has been carried out. 117 Lithiation occurs preferentially
anti to the N-substituent and with a preference for abstraction of an axial hydrogen.
preferred
hydrogen
H
N
R
If the amine carries a chelating substituent, as for 2-methoxyethylamine, the rate of
deprotonation is accelerated. For any specific imine, ring substituents also influence
the imine conformation and rate of deprotonation. These relationships reflect steric,
stereoelectronic, and chelation influences, and sorting out each contribution can be
challenging.
One of the potentially most useful aspects of the imine anions is that they can
be prepared from enantiomerically pure amines. When imines derived from chiral
amines are alkylated, the new carbon-carbon bond is formed with a bias for one of
the two possible stereochemical configurations. Hydrolysis of the imine then leads
to enantiomerically enriched ketone. Table 1.4 lists some examples that have been
reported. 118
The interpretation and prediction of the relationship between the configuration of
the newly formed chiral center and the configuration of the amine is usually based on
steric differentiation of the two faces of the imine anion. Most imine anions that show
high stereoselectivity incorporate a substituent that can engage the metal cation in a
116
M. P. Bernstein and D. B. Collum, J. Am. Chem. Soc., 115, 8008 (1993).
117 S. Liao and D. B. Collum, J. Am. Chem. Soc., 125, 15114 (2003).
118
For a review, see D. E. Bergbreiter and M. Newcomb, in Asymmetric Synthesis, Vol. 2, J. D. Morrison,
ed., Academic Press, New York, 1983, Chap. 9.