Page 76 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 76
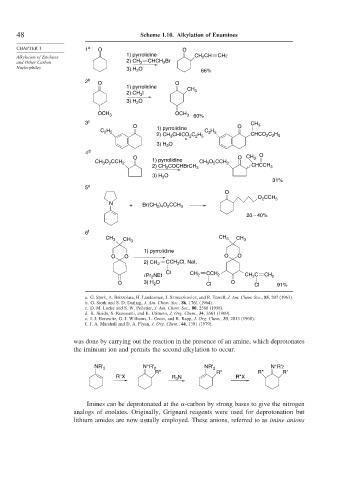
48 Scheme 1.10. Alkylation of Enamines
CHAPTER 1 1 a O O
1) pyrrolidine CH 2 CH
Alkylation of Enolates CH2
and Other Carbon 2) CH CHCH 2 Br
2
Nucleophiles 3) H 2 O 66%
2 b O O
1) pyrrolidine
CH 3
2) CH 3 I
3) H 2 O
OCH 3 OCH 3 60%
3 c
O 1) pyrrolidine O CH 3
C 2 H 5 C 2 H 5
2) CH 3 CHICO 2 C 2 H 5 CHCO 2 C 2 H 5
3) H 2 O
4 d O
O O CH 3
CH 3 O 2 CCH 2 1) pyrrolidine CH 3 O 2 CCH 2
2) CH 3 COCHBrCH 3 CHCCH 3
O
3) H 2
31%
5 e
O
O 2 CCH 3
N
+ Br(CH 2 ) 4 O 2 CCH 3
20 – 40%
6 f
CH 3 CH 3 CH 3
CH 3
1) pyrrolidine
O O O O
CCH 2 Cl, NaI,
2) CH 2
Cl
i Pr NEt CH 2 CCH 2 CH 2 C CH 2
2
O 3) H 2 O Cl O Cl 91%
a. G. Stork, A. Brizzolara, H. Landesman, J. Szmuszkovicz, and R. Terrell, J. Am. Chem. Soc., 85, 207 (1963).
b. G. Stork and S. D. Darling, J. Am. Chem. Soc., 86, 1761 (1964).
c. D. M. Locke and S. W. Pelletier, J. Am. Chem. Soc., 80, 2588 (1958).
d. K. Sisido, S. Kurozumi, and K. Utimoto, J. Org. Chem., 34, 2661 (1969).
e. I. J. Borowitz, G. J. Williams, L. Gross, and R. Rapp, J. Org. Chem., 33, 2013 (1968).
f. J. A. Marshall and D. A. Flynn, J. Org. Chem., 44, 1391 (1979).
was done by carrying out the reaction in the presence of an amine, which deprotonates
the iminium ion and permits the second alkylation to occur.
+
+
NR' 2 N R' 2 NR' 2 N R'2
R" R" R" R"
R"X R 3 N R"X
Imines can be deprotonated at the -carbon by strong bases to give the nitrogen
analogs of enolates. Originally, Grignard reagents were used for deprotonation but
lithium amides are now usually employed. These anions, referred to as imine anions