Page 73 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 73
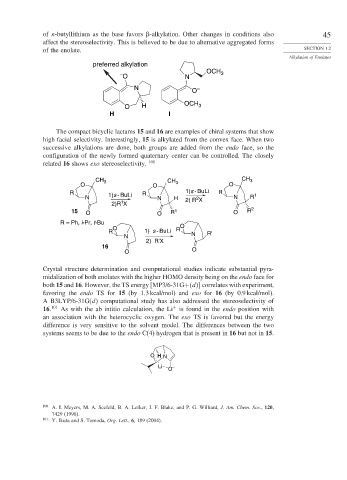
of n-butyllithium as the base favors -alkylation. Other changes in conditions also 45
affect the stereoselectivity. This is believed to be due to alternative aggregated forms
of the enolate. SECTION 1.2
Alkylation of Enolates
preferred alkylation
OCH
– 3
O N
N –
O
OCH
O H 3
H I
The compact bicyclic lactams 15 and 16 are examples of chiral systems that show
high facial selectivity. Interestingly, 15 is alkylated from the convex face. When two
successive alkylations are done, both groups are added from the endo face, so the
configuration of the newly formed quaternary center can be controlled. The closely
related 16 shows exo stereoselectivity. 100
CH 3 CH 3 CH 3
O O O
R 1)s - BuLi R 1)s - BuLi R 1
N N H 2) R X N R
2
1
2)R X
15 O O R 1 O R 2
R = Ph, i-Pr, t-Bu
O R O
R 1) s - BuLi R'
N N
2) R'X
16 O
O
Crystal structure determination and computational studies indicate substantial pyra-
midalization of both enolates with the higher HOMO density being on the endo face for
both 15 and 16. However, the TS energy [MP3/6-31G+ d ] correlates with experiment,
favoring the endo TS for 15 (by 1.3 kcal/mol) and exo for 16 (by 0.9 kcal/mol).
A B3LYP/6-31G(d) computational study has also addressed the stereoselectivity of
+
16. 101 As with the ab intitio calculation, the Li is found in the endo position with
an association with the heterocyclic oxygen. The exo TS is favored but the energy
difference is very sensitive to the solvent model. The differences between the two
systems seems to be due to the endo C(4) hydrogen that is present in 16 but not in 15.
O H N
Li –
O
100 A. I. Meyers, M. A. Seefeld, B. A. Lefker, J. F. Blake, and P. G. Williard, J. Am. Chem. Soc., 120,
7429 (1998).
101
Y. Ikuta and S. Tomoda, Org. Lett., 6, 189 (2004).