Page 72 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 72
44 Scheme 1.9. (Continued)
CHAPTER 1 Li +
– O
Alkylation of Enolates O O O
and Other Carbon f Ph PhCH SCH Br Ph
2
2
Nucleophiles 6 N O N O 83%
PhCH SCH 2
2
)
CH(CH 3 2 CH(CH ) 98:2dr
3 2
O O 1) LiHMDS O O
THF, – 78°C
O N CH 3 O N CH 3
7 g 2) PhCH 2 Br
CH Ph
CH 3 CH 3 CH CH Ph 2 94%
CH 3 CH Ph 3 2
2
94:6dr
O CH 3 O CH 3
CH 3 Ph 1) LDA, LiCl PhCH O Ph
2
8 h N N
2) PhCH OCH CH I CH
2
2
2
CH 3 OH 3 CH 3 OH
Li +
CH 3 CH 3 O – O CH 3 CH 3 O O
CH I PhCH O
3
9 h PhCH O N O 2 N O
2
CH 3
Ph Ph 64%, 3.6:1dr
a. D. A. Evans, M. D. Ennis, and D. J. Mathre, J. Am. Chem. Soc., 104, 1737 (1982).
b. A. Fadel, Synlett, 48 (1992).
c. J. L. Charlton and G-L. Chee, Can. J. Chem., 75, 1076 (1997).
d. C. P. Decicco, D. J. Nelson, B. L. Corbett, and J. C. Dreabit, J. Org. Chem., 60, 4782 (1995).
e. R. P. Beckett, M. J. Crimmin, M. H. Davis, and Z. Spavold, Synlett, 137 (1993).
f. D. A. Evans, D. J. Mathre, and W. L. Scott, J. Org. Chem., 50, 1830 (1985).
g. S. D. Bull, S. G. Davies, R. L. Nicholson, H. J. Sanganee, and A. D. Smith, Organic and Biomolec. Chem., 1,
2886 (2003).
h. J. D. White, C.-S. Lee and Q. Xu, Chem. Commun. 2012 (2003).
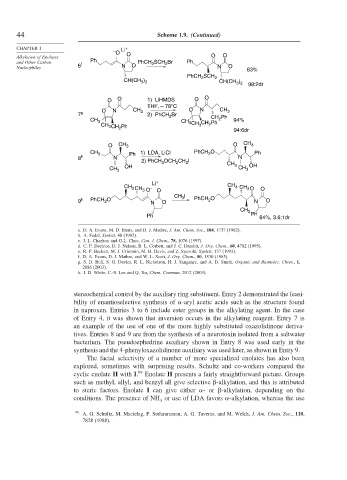
stereochemical control by the auxiliary ring substituent. Entry 2 demonstrated the feasi-
bility of enantioselective synthesis of -aryl acetic acids such as the structure found
in naproxen. Entries 3 to 6 include ester groups in the alkylating agent. In the case
of Entry 4, it was shown that inversion occurs in the alkylating reagent. Entry 7 is
an example of the use of one of the more highly substituted oxazolidinone deriva-
tives. Entries 8 and 9 are from the synthesis of a neurotoxin isolated from a saltwater
bacterium. The pseudoephedrine auxiliary shown in Entry 8 was used early in the
synthesis and the 4-phenyloxazolidinone auxiliary was used later, as shown in Entry 9.
The facial selectivity of a number of more specialized enolates has also been
explored, sometimes with surprising results. Schultz and co-workers compared the
cyclic enolate H with I. 99 Enolate H presents a fairly straightforward picture. Groups
such as methyl, allyl, and benzyl all give selective -alkylation, and this is attributed
to steric factors. Enolate I can give either -or -alkylation, depending on the
conditions. The presence of NH or use of LDA favors -alkylation, whereas the use
3
99
A. G. Schultz, M. Macielag, P. Sudararaman, A. G. Taveras, and M. Welch, J. Am. Chem. Soc., 110,
7828 (1988).