Page 67 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 67
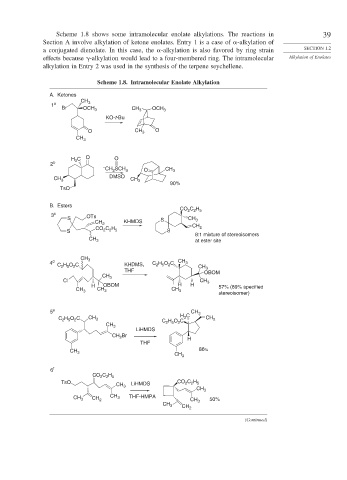
Scheme 1.8 shows some intramolecular enolate alkylations. The reactions in 39
Section A involve alkylation of ketone enolates. Entry 1 is a case of -alkylation of
a conjugated dienolate. In this case, the -alkylation is also favored by ring strain SECTION 1.2
effects because -alkylation would lead to a four-membered ring. The intramolecular Alkylation of Enolates
alkylation in Entry 2 was used in the synthesis of the terpene seychellene.
Scheme 1.8. Intramolecular Enolate Alkylation
A. Ketones
1 a CH 3
Br OCH 3 CH 3 OCH 3
KO-t-Bu
O CH 3 O
CH 3
H 3 C O O
2 b
–
CH 2 SCH 3 O CH 3
DMSO
CH 3 CH 3
90%
TsO
B. Esters
CO 2 C 2 H 5
3 c OTs
S S CH 3
CH 2 KHMDS
CH 2
S CO 2 C 2 H 5 S
8:1 mixture of stereoisomers
CH 3 at ester site
CH 3
4 d C 2 H 5 O 2 C KHDMS, C 2 H 5 O 2 C CH 3
CH 3
THF
OBOM
CH 3
Cl CH 3
H OBOM H H 57% (89% specified
CH 3 CH 3 CH 3
stereoisomer)
5 e CH 3
C 2 H 5 O 2 C CH 3 H 3 C CH 2
C 2 H 5 O 2 C
CH 3
LiHMDS
CH 2 Br
H
THF
86%
CH 3
CH 3
6 f
CO 2 C 2 H 5
TsO LiHMDS CO 2 C 2 H 5
CH 3
CH 3
CH 3 THF-HMPA
CH 3 CH 2 50%
CH 3
CH 3
CH 2
(Continued)