Page 63 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 63
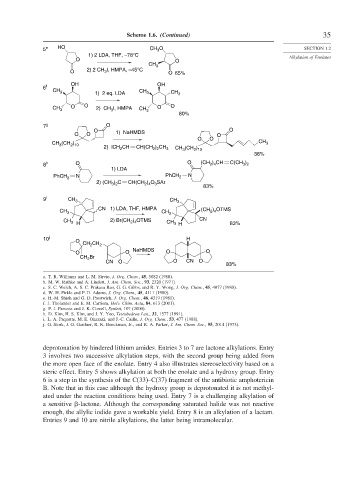
Scheme 1.6. (Continued) 35
5 e HO CH O SECTION 1.2
3
1) 2 LDA, THF, –78°C Alkylation of Enolates
O O
CH 3
O 2) 2 CH 3 I, HMPA, –45°C O 65%
OH OH
6 f
CH 3 1) 2 eq. LDA CH 3 CH 3
CH 3 O O 2) CH I, HMPA CH 3 O O
3
80%
7 g O
O O
O O 1) NaHMDS O
O O
CH (CH ) CH 3
2 10
3
2) ICH 2 CH CH(CH ) CH 3 CH (CH )
2 2
2 10
3
36%
3 2
2 4
8 h O O (CH ) CH C(CH )
1) LDA
PhCH 2 N PhCH 2 N
2) (CH ) C CH(CH ) O SAr
3
3 2
2 4
83%
9 i CH 3 CH 3
) OTMS
CH 3 CN 1) LDA, THF, HMPA CH 3 (CH 2 4
CH 3 H 2) Br(CH ) OTMS CH 3 H CN 83%
2 4
10 j H
O
CH CH 2
2
O O NaHMDS O O
Br
CH 2
CN O O CN O
83%
a. T. R. Williams and L. M. Sirvio, J. Org. Chem., 45, 5082 (1980).
b. M. W. Rathke and A. Lindert, J. Am. Chem. Soc., 93, 2320 (1971).
c. S. C. Welch, A. S. C. Prakasa Rao, G. G. Gibbs, and R. Y. Wong, J. Org. Chem., 45, 4077 (1980).
d. W. H. Pirkle and P. E. Adams, J. Org. Chem., 45, 4111 (1980).
e. H.-M. Shieh and G. D. Prestwich, J. Org. Chem., 46, 4319 (1981).
f. J. Tholander and E. M. Carriera, Helv. Chim. Acta, 84, 613 (2001).
g. P. J. Parsons and J. K. Cowell, Synlett, 107 (2000).
h. D. Kim, H. S. Kim, and J. Y. Yoo, Tetrahedron Lett., 32, 1577 (1991).
i. L. A. Paquette, M. E. Okazaki, and J.-C. Caille, J. Org. Chem., 53, 477 (1988).
j. G. Stork, J. O. Gardner, R. K. Boeckman, Jr., and K. A. Parker, J. Am. Chem. Soc., 95, 2014 (1973).
deprotonation by hindered lithium amides. Entries 3 to 7 are lactone alkylations. Entry
3 involves two successive alkylation steps, with the second group being added from
the more open face of the enolate. Entry 4 also illustrates stereoselectivity based on a
steric effect. Entry 5 shows alkylation at both the enolate and a hydroxy group. Entry
6 is a step in the synthesis of the C(33)–C(37) fragment of the antibiotic amphotericin
B. Note that in this case although the hydroxy group is deprotonated it is not methyl-
ated under the reaction conditions being used. Entry 7 is a challenging alkylation of
a sensitive -lactone. Although the corresponding saturated halide was not reactive
enough, the allylic iodide gave a workable yield. Entry 8 is an alkylation of a lactam.
Entries 9 and 10 are nitrile alkylations, the latter being intramolecular.