Page 59 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 59
The selectivity for electrophilic attack at the -carbon presumably reflects a greater 31
negative charge, as compared with the -carbon.
SECTION 1.2
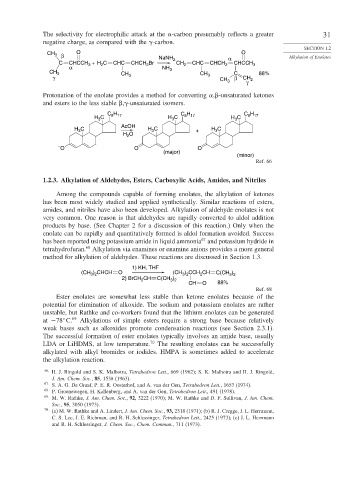
CH 3 β O O
NaNH 2 α Alkylation of Enolates
C CHCCH + H C CHC CHCH Br CH 2 CHC CHCH 2 CHCCH 3
2
3
2
α NH
CH 3 CH 3 CH C 88%
γ 3 3 CH 3 β CH 2
γ
Protonation of the enolate provides a method for converting , -unsaturated ketones
and esters to the less stable , -unsaturated isomers.
H H H
C 8 17 C 8 17 C 8 17
H C H C H C
3
3
3
AcOH
C H C H C
H 3 3 + 3
H O
2
– O O O
(major)
(minor)
Ref. 66
1.2.3. Alkylation of Aldehydes, Esters, Carboxylic Acids, Amides, and Nitriles
Among the compounds capable of forming enolates, the alkylation of ketones
has been most widely studied and applied synthetically. Similar reactions of esters,
amides, and nitriles have also been developed. Alkylation of aldehyde enolates is not
very common. One reason is that aldehydes are rapidly converted to aldol addition
products by base. (See Chapter 2 for a discussion of this reaction.) Only when the
enolate can be rapidly and quantitatively formed is aldol formation avoided. Success
67
has been reported using potassium amide in liquid ammonia and potassium hydride in
68
tetrahydrofuran. Alkylation via enamines or enamine anions provides a more general
method for alkylation of aldehydes. These reactions are discussed in Section 1.3.
1) KH, THF
(CH ) CHCH O (CH ) CCH CH C(CH )
3 2
3 2
2
3 2
2) BrCH CH C(CH )
2
3 2
CH O 88%
Ref. 68
Ester enolates are somewhat less stable than ketone enolates because of the
potential for elimination of alkoxide. The sodium and potassium enolates are rather
unstable, but Rathke and co-workers found that the lithium enolates can be generated
at −78 C. 69 Alkylations of simple esters require a strong base because relatively
weak bases such as alkoxides promote condensation reactions (see Section 2.3.1).
The successful formation of ester enolates typically involves an amide base, usually
LDA or LiHDMS, at low temperature. 70 The resulting enolates can be successfully
alkylated with alkyl bromides or iodides. HMPA is sometimes added to accelerate
the alkylation reaction.
66
H. J. Ringold and S. K. Malhotra, Tetrahedron Lett., 669 (1962); S. K. Malhotra and H. J. Ringold,
J. Am. Chem. Soc., 85, 1538 (1963).
67
S. A. G. De Graaf, P. E. R. Oosterhof, and A. van der Gen, Tetrahedron Lett., 1653 (1974).
68 P. Groenewegen, H. Kallenberg, and A. van der Gen, Tetrahedron Lett., 491 (1978).
69 M. W. Rathke, J. Am. Chem. Soc., 92, 3222 (1970); M. W. Rathke and D. F. Sullivan, J. Am. Chem.
Soc., 95, 3050 (1973).
70
(a) M. W. Rathke and A. Lindert, J. Am. Chem. Soc., 93, 2318 (1971); (b) R. J. Cregge, J. L. Herrmann,
C. S. Lee, J. E. Richman, and R. H. Schlessinger, Tetrahedron Lett., 2425 (1973); (c) J. L. Herrmann
and R. H. Schlessinger, J. Chem. Soc., Chem. Commun., 711 (1973).