Page 55 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 55
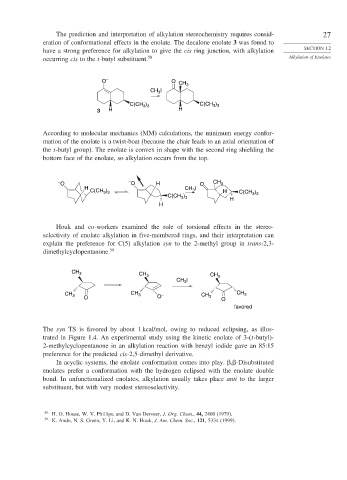
The prediction and interpretation of alkylation stereochemistry requires consid- 27
eration of conformational effects in the enolate. The decalone enolate 3 was found to
have a strong preference for alkylation to give the cis ring junction, with alkylation SECTION 1.2
occurring cis to the t-butyl substituent. 58 Alkylation of Enolates
O – O CH 3
CH I
3
C(CH ) C(CH )
3 3
3 3
3 H H
According to molecular mechanics (MM) calculations, the minimum energy confor-
mation of the enolate is a twist-boat (because the chair leads to an axial orientation of
the t-butyl group). The enolate is convex in shape with the second ring shielding the
bottom face of the enolate, so alkylation occurs from the top.
– O – O H O CH 3
H CH I
C(CH ) 3 H C(CH )
3 3
C(CH ) 3 3
3 3
H
H
Houk and co-workers examined the role of torsional effects in the stereo-
selectivity of enolate alkylation in five-membered rings, and their interpretation can
explain the preference for C(5) alkylation syn to the 2-methyl group in trans-2,3-
dimethylcyclopentanone. 59
CH 3 CH 3 CH
CH I 3
3
CH 3 CH 3 – CH CH 3
O O 3 O
favored
The syn TS is favored by about 1 kcal/mol, owing to reduced eclipsing, as illus-
trated in Figure 1.4. An experimental study using the kinetic enolate of 3-(t-butyl)-
2-methylcyclopentanone in an alkylation reaction with benzyl iodide gave an 85:15
preference for the predicted cis-2,5-dimethyl derivative.
In acyclic systems, the enolate conformation comes into play. , -Disubstituted
enolates prefer a conformation with the hydrogen eclipsed with the enolate double
bond. In unfunctionalized enolates, alkylation usually takes place anti to the larger
substituent, but with very modest stereoselectivity.
58 H. O. House, W. V. Phillips, and D. Van Derveer, J. Org. Chem., 44, 2400 (1979).
59
K. Ando, N. S. Green, Y. Li, and K. N. Houk, J. Am. Chem. Soc., 121, 5334 (1999).