Page 60 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 60
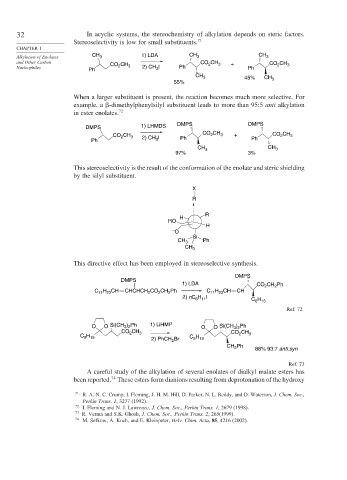
32 In acyclic systems, the stereochemistry of alkylation depends on steric factors.
Stereoselectivity is low for small substituents. 71
CHAPTER 1
1) LDA CH
Alkylation of Enolates CH 3 CH 3 3
and Other Carbon CO CH CO CH 3 + CO CH 3
2
2
Nucleophiles Ph 2 3 2) CH 3 I Ph Ph
CH 3 45% CH
55% 3
When a larger substituent is present, the reaction becomes much more selective. For
example, a -dimethylphenylsilyl substituent leads to more than 95:5 anti alkylation
in ester enolates. 72
DMPS 1) LHMDS DMPS DMPS
CH
CH CO 2 3 + CH
CO 2 3 2) CH I CO 2 3
Ph 3 Ph Ph
CH
CH 3 3
97% 3%
This stereoselectivity is the result of the conformation of the enolate and steric shielding
by the silyl substituent.
X
R
R
H
RO
H
– O
Si
CH 3 Ph
CH 3
This directive effect has been employed in stereoselective synthesis.
DMPS
DMPS
1) LDA CO CH Ph
2
2
C H CH CHCHCH CO CH Ph C H CH CH
11 23
11 23
2
2
2
H I
C H
2) nC 6 11
6 13
Ref. 72
O O Si(CH ) Ph 1) LiHMP O O Si(CH ) Ph
3 2
3 2
CO CH 3 CO CH
2
C H C H 2 3
9 19
2) PhCH 2 Br 9 19
CH Ph 88% 93:7 anti:syn
2
Ref. 73
A careful study of the alkylation of several enolates of dialkyl malate esters has
74
been reported. These esters form dianions resulting from deprotonation of the hydroxy
71 R. A. N. C. Crump, I. Fleming, J. H. M. Hill, D. Parker, N. L. Reddy, and D. Waterson, J. Chem. Soc.,
Perkin Trans. 1, 3277 (1992).
72
I. Fleming and N. J. Lawrence, J. Chem. Soc., Perkin Trans. 1, 2679 (1998).
73 R. Verma and S.K. Ghosh, J. Chem. Soc., Perkin Trans. 2, 265(1999).
74
M. Sefkow, A. Koch, and E. Kleinpeter, Helv. Chim. Acta, 85, 4216 (2002).