Page 57 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 57
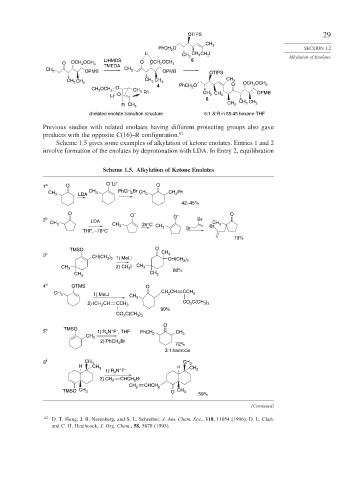
OTIPS 29
CH 3
PhCH O SECTION 1.2
2
Li CH CH CH I
3 3 2 Alkylation of Enolates
LiHMDS 5
O OCH OCH 3 O OCH OCH 3
2
2
TMEDA
OPMB OPMB OTIPS
CH 3 CH 3
CH 3 CH CH CH CH 3
3
3 4 3 PhCH O O OCH OCH 3
2
CH OCH O CH 2
3 2 3 R'I CH CH OPMB
Li O 3 3
6 CH CH
R CH 3 CH 3 3 3
chelated enolate transition structure 6:1 S:R in 55:45 hexane-THF
Previous studies with related enolates having different protecting groups also gave
products with the opposite C(16)–R configuration. 62
Scheme 1.5 gives some examples of alkylation of ketone enolates. Entries 1 and 2
involve formation of the enolates by deprotonation with LDA. In Entry 2, equilibration
Scheme 1.5. Alkylation of Ketone Enolates
–
1 a O O Li + O
CH 3 LDA CH 3 PhCH 2 Br CH 3 CH Ph
2
42–45%
O O – – O
2 b LDA O Br
CH 3 CH 3 25°C CH Br CH 3
THF, –78°C 3 Br
79%
TMSO O
3
3 c CH(CH ) CH CH(CH )
3 2 1) MeLi
3 2
CH 3 2) CH I CH 3
3
CH 80%
CH 3 3
4 d OTMS O
CH 3 1) MeLi CH 3 CH CH CCH 3
2
2) ICH CH CCH CO C(CH )
2
3 3
2 3
90%
CO C(CH )
3 3
2
O
+ –
5 e TMSO 1) R N F , THF PhCH 2 CH
4
CH 3 3
Br
2) PhCH 2
72%
3:1 trans:cis
6 f CH 3 CH 3
H CH 3 H CH
+ –
1) R N F 3
4
CHCH 2 Br
2) CH 2
CH 2 CHCH 2
TMSO CH 3 O CH 3
59%
(Continued)
62
D. T. Hung, J. B. Nerenberg, and S. L. Schreiber, J. Am. Chem. Soc., 118, 11054 (1996); D. L. Clark
and C. H. Heathcock, J. Org. Chem., 58, 5878 (1993).