Page 53 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 53
52
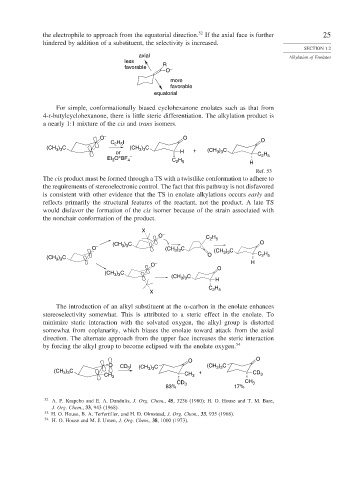
the electrophile to approach from the equatorial direction. If the axial face is further 25
hindered by addition of a substituent, the selectivity is increased.
SECTION 1.2
axial Alkylation of Enolates
less
R
favorable
O –
more
favorable
equatorial
For simple, conformationally biased cyclohexanone enolates such as that from
4-t-butylcyclohexanone, there is little steric differentiation. The alkylation product is
a nearly 1:1 mixture of the cis and trans isomers.
O – O
C H I O
2 5
(CH ) C (CH ) C
3 3
3 3
3 3
or H + (CH ) C H
+
Et O BF 4 – H C 2 5
3
C 2 5
H
Ref. 53
The cis product must be formed through a TS with a twistlike conformation to adhere to
the requirements of stereoelectronic control. The fact that this pathway is not disfavored
is consistent with other evidence that the TS in enolate alkylations occurs early and
reflects primarily the structural features of the reactant, not the product. A late TS
would disfavor the formation of the cis isomer because of the strain associated with
the nonchair conformation of the product.
X
O – C H
2 5
) C
(CH 3 3 O
O – (CH ) C (CH ) C
3 3
O 3 3 C H
2 5
(CH ) C
3 3
O – H
O
(CH ) C (CH ) C H
3 3
3 3
C H
X 2 5
The introduction of an alkyl substituent at the -carbon in the enolate enhances
stereoselectivity somewhat. This is attributed to a steric effect in the enolate. To
minimize steric interaction with the solvated oxygen, the alkyl group is distorted
somewhat from coplanarity, which biases the enolate toward attack from the axial
direction. The alternate approach from the upper face increases the steric interaction
by forcing the alkyl group to become eclipsed with the enolate oxygen. 54
O O
O CD I (CH ) C (CH ) C
3 3
(CH 3 ) 3 C 3 3 3 +
CH 3 CH 3 CD 3
CD CH 3
83% 3 17%
52 A. P. Krapcho and E. A. Dundulis, J. Org. Chem., 45, 3236 (1980); H. O. House and T. M. Bare,
J. Org. Chem., 33, 943 (1968).
53 H. O. House, B. A. Terfertiller, and H. D. Olmstead, J. Org. Chem., 33, 935 (1968).
54
H. O. House and M. J. Umen, J. Org. Chem., 38, 1000 (1973).