Page 48 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 48
20 the hexameric clusters are a good indication of the nature of the enolates in relatively
weakly coordinating solvents. In both structures, series of alternating metal cations
CHAPTER 1
and enolate oxygens are assembled in two offset hexagons. The cluster is considerably
Alkylation of Enolates tighter with Li than with K . The M−O bonds are about 1.9 Å for Li and 2.6 Å for
+
+
+
and Other Carbon
+
+
+
Nucleophiles K . The enolate C−O bond is longer (1.34 Å) for Li than for K (1.31 Å), whereas
+
+
+
the C=C bond is shorter for Li (1.33 Å) than for K (1.35 Å). Thus, the Li enolate
has somewhat more of oxy-anion character and is expected to be a “harder” than the
potassium enolate.
Despite the somewhat reduced reactivity of aggregated enolates, THF and DME
are the most commonly used solvents for synthetic reactions involving enolate
alkylation. They are the most suitable solvents for kinetic enolate generation and also
have advantages in terms of product workup and purification over the polar aprotic
solvents. Enolate reactivity in these solvents can often be enhanced by adding a reagent
that can bind alkali metal cations more strongly. Popular choices are HMPA, DMPU,
tetramethylethylenediamine (TMEDA), and the crown ethers. TMEDA chelates metal
ions through the electron pairs on nitrogen. The crown ethers encapsulate the metal
ions through coordination with the ether oxygens. The 18-crown-6 structure is of such
a size as to allow sodium or potassium ions to fit in the cavity. The smaller 12-crown-4
+
binds Li preferentially. The cation complexing agents lower the degree of aggregation
of the enolate and metal cations, which results in enhanced reactivity.
The effect of HMPA on the reactivity of cyclopentanone enolate has been
examined. 44 This enolate is primarily a dimer, even in the presence of excess HMPA,
but the reactivity increases by a factor of 7500 for a tenfold excess of HMPA at −50 C.
The kinetics of the reaction with CH I are consistent with the dimer being the active
3
nucleophile. It should be kept in mind that the reactivity of regio- and stereoisomeric
enolates may be different and the alkylation product ratio may not reflect the enolate
composition. This issue was studied with 2-heptanone. 45 Although kinetic deproton-
ation in THF favors the 1-enolate, a nearly equal mixture of C(1) and C(3) alkylation
was observed. The inclusion of HMPA improved the C(1) selectivity to 11:1 and also
markedly accelerated the rate of the reaction. These results are presumably due to
increased reactivity and less competition from enolate isomerization in the presence
of HMPA.
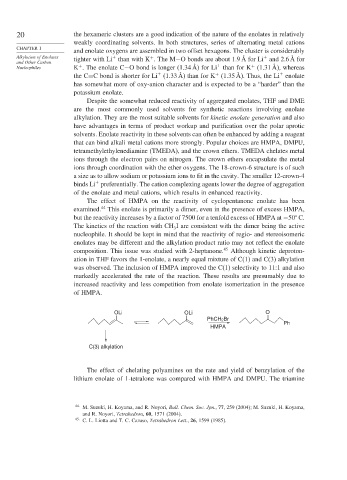
OLi OLi O
PhCH 2Br
Ph
HMPA
C(3) alkylation
The effect of chelating polyamines on the rate and yield of benzylation of the
lithium enolate of 1-tetralone was compared with HMPA and DMPU. The triamine
44 M. Suzuki, H. Koyama, and R. Noyori, Bull. Chem. Soc. Jpn., 77, 259 (2004); M. Suzuki, H. Koyama,
and R. Noyori, Tetrahedron, 60, 1571 (2004).
45
C. L. Liotta and T. C. Caruso, Tetrahedron Lett., 26, 1599 (1985).